
Management of relapsed and refractory mantle cell lymphoma: a review of current evidence and future directions for research
Introduction
Mantle cell lymphoma (MCL) is a rare subtype of non-Hodgkin’s lymphoma (NHL) comprising less than 10% of NHL, characterized cytogenetically by translocation (11;14 ) (q13,q32) resulting in overexpression of cyclin D1 and clinically by a heterogeneous but often aggressive disease course (1-4). The median age at diagnosis is between 60–66 years with men affected more frequently than women by a ratio of more than 2:1 (5-8). While high response rates are seen with induction chemo-immunotherapy, relapse is almost universal, occurring linearly even beyond 6 years from end of therapy (9). Management of relapsed disease is challenging, with treatment decisions based on patient and disease characteristics.
In this review we will review in depth the management of relapsed/refractory MCL. We will discuss data from recent and ongoing clinical trials and future directions for investigation.
Relapsed and refractory MCL, current management
For patients with relapsed MCL, choice of therapy depends upon multiple factors including prior therapy, duration of response (DOR), and patient characteristics such as fitness and comorbidities which impact the ability to tolerate hematopoietic cell transplant (HCT). There is a subset of patients with MCL with indolent disease biology and primarily non nodal disease (10-12). Observation has been shown to be safe in selected cases at initial diagnosis in retrospective studies (13,14) and may have a role in patients with indolent disease at relapse. However, therapy is warranted at relapse in the majority of cases due to the aggressive clinical nature of MCL.
Intensive combination chemo immunotherapy for transplant eligible patients
For fit patients who are ASCT candidates and have not previously undergone ASCT consolidation or for patients with relapse after ASCT deemed to be candidates for allogeneic HCT, intensive chemo-immunotherapy should be considered as a bridge to transplant. Intensive cytarabine containing regimens including R-DHAP (rituximab plus dexamethasone, cytarabine, and cisplatin), R-ESHAP (rituximab plus etoposide, methylprednisolone, cytarabine, and cisplatin), or R-BAC (rituximab plus bendamustine and cytarabine) are options for relapsed disease (15-18). BR (bendamustine and rituximab) is an alternative option, particularly for patients not previously treated with bendamustine, which had a 50% CR and 75% overall response rate (ORR) in patients with relapsed MCL treated as part of a phase II study (19). R-ICE (rituximab plus ifosfamide, carboplatin, and etoposide) is an alternative regimen that has also been used for relapsed MCL as salvage therapy prior to transplant (20,21).
The evidence for ASCT for patients with relapsed disease comes primarily from retrospective studies including MCL patients transplanted both as consolidation in first CR (CR1) and with relapsed disease. Vandenberghe et al. reported outcomes for 195 MCL patients undergoing ASCT with variable conditioning regimens with 50% OS and 33% PFS at 5 years, but noted that patients in CR1 fared significantly better than patients with relapsed/refractory disease (hazard ratio 2.9) (22). Till et al. again noted superior outcomes for patients receiving ASCT for MCL in CR1, but noted a 3-year PFS of 63% in relapsed chemosensitive disease and 35% with refractory disease prior to transplant (23). Fenske et al. reported a 29% PFS and 44% OS at 5 years for 132 MCL patients receiving ASCT following disease relapse (24). Thus, while ASCT for patients with relapsed chemosensitive disease offers less benefit compared with ASCT as initial consolidation, it does offer durable disease control for a minority of patients. Cassaday et al. retrospectively assessed 67 patients with relapsed/refractory MCL undergoing ASCT at a single institution and identified absence of B symptoms at diagnosis, low risk MIPI score, longer time from diagnosis, and fewer prior lines of therapy as predictive of more durable response (25). Patients with favorable risk factors demonstrated a 55% 5-year PFS suggesting risk stratification can aid in selecting patients most likely to achieve meaningful benefit from ASCT (25).
For relapsed MCL following prior ASCT, allogeneic HCT historically has offered the most durable remissions for suitable patients (26-28). Evidence for HCT in MCL comes from a limited number of prospective studies as well as retrospective series and registry studies with considerable variation in reported outcomes (27,29,30). Vaughn et al. reported a 10-year OS of 44% and 41% PFS for patients receiving non-myeloablative allogeneic HCT for MCL at the Fred Hutchinson Cancer Center, while Tam et al reported 6-year OS of 46% and 53% PFS at the M.D. Anderson Cancer Center with non-myeloablative allogeneic HCT (27,31). Other series reported less encouraging results which may be due in part to differences in disease status of patients at time of transplant, including Cook et al who reported 5-year OS of 37% and PFS of 14% (32). Non-myeloablative conditioning appears to be the preferred approach for patients with MCL with a recent meta-analysis demonstrating lower non relapse mortality (NRM) as well as superior OS and PFS compared with ablative regimens (33). However, NRM still contributes significantly to mortality in patients undergoing non-myeloablative HCT with reported rates ranging from 18–32% (27,30-32,34-36).
HCT appears to have the highest likelihood of success in patients with chemo-sensitive disease, but patients with refractory disease have been treated successfully. Hamadani et al. reported outcomes for patients with chemotherapy refractory aggressive NHL including MCL treated with HCT and noted a 5-year PFS and OS of 46% of patients with stable disease prior to transplant compared with 21% PFS and 7% OS for patients with progressive disease following their antecedent therapy (37). Hamadani et al. reported outcomes for 202 patients with chemoresistant MCL from the Center for International Blood and Marrow Transplant Research database and found that allogeneic transplant was associated with durable remission in 20–25% of patients at three years (38). NRM was high at 45% overall; there was no significant difference observed between choice of myeloablative and non-myeloablative conditioning and NRM, PFS, or OS in this series (38).
Chemo-immunotherapy for patients who are not optimal candidates for transplant
For patients who are not good candidates for intensive therapy with the goal of proceeding to transplant due to comorbidities, fitness, disease behavior, or patient preference, multiple options for treatment are available including chemo-immunotherapy. Results from trials of chemoimmunotherapy are summarized in Table 1. The choice of therapy depends upon disease characteristics including prior therapies received and DOR achieved and patient characteristics including comorbidities and PS. For patients without prior treatment with bendamustine or with a previous prolonged response to bendamustine, BR or single agent bendamustine for patients with rituximab refractory disease are preferred options with ORR ranging from 75–93% in phase II studies and median progression free survival (PFS) ranging from 17–18 months (19,39,46). A phase II trial of R-BAC demonstrated 80% ORR, including 70% CR, and a 70% 2-year PFS (17); however, hematologic toxicity was significant including 57% of patients with grade 3/4 leukopenia and 87% grade 3/4 thrombocytopenia. Less hematologic toxicity was seen in a subsequent study of R-BAC for previously untreated MCL using a lower dosage of cytarabine (17,47). Ibrutinib has been added to BR with promising efficacy and an acceptable safety profile warranting further study (48). Gemcitabine has modest single agent activity against MCL with an ORR of 28% (41), and higher response rates have been shown in phase II studies in combination with platinum agents (42,43). Although rituximab, gemcitabine and cisplatin has not been directly compared with rituximab, gemcitabine, and oxaliplatin (RGemOx), tolerability may be better with RGemOx with less nausea and nephrotoxicity seen with oxaliplatin compared with cisplatin and response rates and DOR appear similar. Fludarabine and cyclophosphamide (FC) with or without rituximab achieved an ORR of 75% for 16 patients with relapsed MCL, but responses were typically not durable with a median DOR of 11 months (45). Fludarabine and rituximab was compared with BR in a phase III trial with an inferior ORR (26%) and PFS (4.7 months) in comparison to BR for patients with MCL (40). CHOP was studied with or without bortezomib in a phase III trial of 46 patients with MCL at first relapse with an ORR of 47% with median PFS of 8.1 months for CHOP alone versus 82.6% ORR and 16.5 months PFS with the addition of bortezomib (44). However, interpretation is limited as the CHOP arm did not include rituximab which improves ORR and time to treatment failure when added to CHOP frontline and improves response rates with cytotoxic chemotherapy in the relapsed setting (49,50).
Table 1
| Regimen | ORR (%); (ref.) | CR rate (%); (ref.) | PFS (months); (ref.) |
|---|---|---|---|
| Bendamustine + R | 71–92; (19,39,40) | 38–50; (19,39,40) | 17.6–18.1; (19,39,40) |
| R-BAC | 80; (17) | 70; (17) | NR, 70% 2-year PFS; (17) |
| Fludarabine + R | 26; (40) | 13; (40) | 4.7; (40) |
| Gemcitabine | 28; (41) | 5.6; (41) | 8.0; (41) |
| Gemcitabine and dex* | 36; (42) | 22; (42) | 3.0; (42) |
| Gemcitabine, dex, and cisplatin** | 85; (42) | 60; (42) | 8.5; (42) |
| R-GemOx | 85; (43) | 60; (43) | 22; (43) |
| CHOP | 47; (44) | 22; (44) | 8.1; 44) |
| V+CHOP | 83; (44) | 35; (44) | 16.5; (44) |
| FC +/− R | 75; (45) | 56; (45) | 11.0; (45) |
*, included only patients aged 70 and older; **, included only patients aged less than 70. ORR, overall response rate; CR, complete response; PFS, progression free survival; R, rituximab; R-BAC, rituximab, bendamustine, and cytarabine; NR, not reached; dex, dexamethasone; R-GemOx, rituximab, gemcitabine, and oxaliplatin; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; V, bortezomib; FC, fludarabine and cyclophosphamide; ref. reference.
Biologic agents for the treatment of relapsed or refractory MCL
Ibrutinib (Imbruvica, Pharmacyclics; Sunnyvale, CA, USA) is an oral inhibitor of B-cell receptor (BCR) signaling through targeting of Bruton’s tyrosine kinase (BTK) which has become the preferred therapy at relapse for a majority of patients. In addition to BTK, ibrutinib also inhibits interleukin-2-inducible kinase mediating a shift towards Th1 mediated immunity as well as off target toxicities (51). A phase I study in relapsed/refractory B-cell malignancies showed a promising 78% ORR among patients with MCL prompting a large phase II study (52).
In the landmark phase II international trial reported by Wang et al., 111 patients with relapsed/refractory MCL were treated with ibrutinib 560 mg daily until disease progression or unacceptable toxicity (53). The median age was 68, 43% had previously received bortezomib, and 86% had intermediate or high-risk disease by MIPI. An ORR of 68% was achieved including 21% CR (53). Adverse events included diarrhea (50%), nausea (31%), edema (28%), anorexia (21%), and rash (19%). Hematologic toxicity included neutropenia in 18% of patients (16% ≥ grade 3), thrombocytopenia in 18% (11% ≥ grade 3), and anemia in 10%. Lymphocytosis was seen in 34% of patients with a peak at 4 weeks, and flow cytometric analysis showed circulating lymphocytes to be primarily CD19 and CD5 positive and CD3 negative consistent with redistribution of MCL cells from lymph nodes into the peripheral circulation. With extended follow-up, median PFS was 13 months with median OS of 22.5 months (54). The median DOR was 17.5 months with 31% 24-month PFS. Patients with refractory rather than relapsed disease at study entry had inferior outcomes with a median OS of 13 months. With extended follow-up, adverse events of interest included atrial fibrillation in 11% and any bleeding events reported in 50% of patients including 6% of patients with ≥ grade 3 events (54). Grade 3 bleeding events included subdural hematoma (2%) and hematuria (2%). A second phase II trial confirmed similar results among 120 patients previously treated with bortezomib and rituximab containing regimens with a 63% ORR including 21% CR and toxicities similar to those previously reported including diarrhea (43%), bleeding (38%), atrial fibrillation (11%), and major bleeding (3%) (55).
Ibrutinib was compared with temsirolimus in a phase III trial of 280 patients with relapsed/refractory MCL. Ibrutinib was associated with a greater ORR (72%, P<0.0001) and CR rate (19%) as well as a significantly longer PFS (14.6 vs. 6.2 months, P<0.0001) (56). Therapy with ibrutinib was better tolerated with fewer dose reductions (4% vs. 43%) and fewer discontinuations due to AE (6% vs. 26%). For an overview of results from phase II and III trials of biologic agents for MCL, refer to Table 2.
Table 2
| Medication | ORR (%); (ref.) | CR (%); (ref.) | PFS (months); (ref.) | Non hematologic serious AE | Rate of discontinuation due to toxicity (%); (ref.) |
|---|---|---|---|---|---|
| Ibrutinib | 68–72; (53,56) | 19-21; (53,56) | 13–14.6; (53,56) | Atrial fibrillation, bleeding, rash, lymphocytosis (53,56) | 6; (56) |
| Lenalidomide | 35–40; (57,58) | 5–12; (57,58) | 8.7–8.8; (57,58) | Rash, diarrhea, fatigue, tumor flare (57,58) | 15; (58) |
| Bortezomib | 32–41; (59,60) | 8–21; (59,60) | 6.5; (59,60) | Neuropathy, hypotension, fatigue, rash, nausea (59,60) | 22; (59) |
| Temsirolimus | 22–40; (56,61) | 1–2; (56,61) | 4.8–6.2; (56,61) | Mucositis, pneumonitis, elevated triglycerides, diarrhea, fever, edema (56) | 26; (56) |
MCL, mantle cell lymphoma; ORR, overall response rate; CR, complete response rate; PFS, median progression free survival; AE, adverse events.
Outcomes after progression on ibrutinib
Published experience to date suggests that patients who discontinue ibrutinib due to disease progression have a grave prognosis with currently available therapies (62-64). Cheah et al. reported outcomes for 42 patients treated with ibrutinib alone or with rituximab who subsequently discontinued therapy, most commonly due to disease progression (39%) including 19% with primary refractory disease (62). The median OS was 8.4 months after ibrutinib discontinuation; 74% of patients received subsequent therapy. The ORR to subsequent therapy was 32% with no therapy associated with superior response. Martin et al reported outcomes for 114 patients across 15 academic medical centers experiencing disease progression while on ibrutinib (63). The median age was 68 and median duration of ibrutinib therapy was 4.7 months. 55% of patients had objective response to ibrutinib prior to disease progression; 35% of patients had a best response of progressive disease. The median OS after discontinuation of ibrutinib was 2.9 months. Following ibrutinib discontinuation, 30% of patients received no subsequent therapy with a median OS of 0.8 months. For the remaining 70%, a 26% ORR was reported with 1.9 month median PFS (63). Further evidence of unsatisfactory outcomes following ibrutinib discontinuation comes from a retrospective series of patients treated for MCL with ibrutinib as standard of care at five US academic medical centers. A total of 49 patients discontinued ibrutinib, with disease progression the reason for discontinuation in 45 of 49 cases; the median OS following discontinuation was 2.5 months (64). Patients with progression after initial response fared better than patients with refractory disease (median OS 5 vs. 1 month) (64). The reason for poor outcomes after ibrutinib discontinuation is unclear and interpretation is limited by the retrospective nature of published series. Patient characteristics including age and number of prior treatments are likely contributory, but whether disease biology is uniquely altered at progression while on ibrutinib is unknown. Patients who discontinue ibrutinib for reasons other than disease progression do not appear to have similarly poor outcomes (65). Given poor outcomes for patients with progressive MCL while on ibrutinib, well-designed clinical trials are urgently needed to address the best approach to treatment. Encouraging response rates (3 PR, 2 CR) were reported with multi-agent therapy (bortezomib, lenalidomide, dexamethasone, and rituximab) in a series of 5 ibrutinib resistant patients warranting further study (66). Given the poor outcomes following progression on ibrutinib, allogeneic HCT is warranted for suitable patients with response to therapy after progression on ibrutinib, and should be considered for patients who are transplant candidates currently responding to ibrutinib.
Bortezomib (Velcade, Millennium Pharmaceuticals, Cambridge, MA, USA) is a proteasome inhibitor which inhibits nuclear factor κ B (NF-κB) signaling pathway through prevention of IκB degradation within the proteasome (67). Targeting NF-κB signaling in MCL cell lines has been shown to induce apoptosis, leading to interest in utilizing bortezomib for therapy in MCL (68). In a phase II study of bortezomib dosed at 1.5 mg/m2 IV, a 40% ORR was seen with 20% CR in patients with MCL (59). In the landmark phase II PINNACLE trial, 155 patients with relapsed/refractory MCL were treated with bortezomib 1.3 mg/m2 IV days 1, 4, 8, and 11 of a 21-day cycle until disease progression or toxicity (69). A 32% ORR was seen including 8% CR, with a median DOR of 9.2 months (60,69). Toxicities included grade 3 neuropathy (13%), fatigue (11%), and thrombocytopenia (10%), with a 3% mortality rate attributed to treatment (69). A phase II study of bortezomib 1.5 mg/m2 IV in combination with rituximab was limited due to high rates of grade 3 or higher neurologic toxicity, and the trial was amended to decrease the dose of bortezomib to 1.3 mg/m2 (70). In a separate phase II trial bortezomib dosed at 1.3 mg/m2 was combined with rituximab and dexamethasone in 16 patients with MCL. An 81% ORR was seen including 44% CR with a median PFS of 12.1 months (71). Grade 3 toxicities included thrombocytopenia (38%), fatigue (19%), and neuropathy (13%). Bortezomib was combined with rituximab and bendamustine in a phase II trial with 71% ORR in MCL and overall 2 year PFS of 47%; 23% of patients were unable to complete all 6 cycles of treatment due to toxicity (72). Finally, bortezomib has been combined with cyclophosphamide in a phase II study in relapsed/refractory MCL (73). Grade 3/4 hematologic toxicity occurred in 25% of cycles, the ORR was 74% with 42% CR and a median PFS of 9 months (73). Overall, combination studies of bortezomib show higher rates of toxicity compared with monotherapy and it remains unclear response rates and DOR are superior in the absence of a comparative trial. In patients with prior treatment-related neuropathy, bortezomib can be difficult to administer.
Lenalidomide (Revlimid, Celgene; Summit, NJ, USA) is a novel oral thalidomide derivative. After demonstrating promising activity with a 53% ORR including 20% CR in a subset of 15 patients with relapsed/refractory MCL enrolled in the NHL-002 pilot study, 57 patients with relapsed/refractory MCL were enrolled in the international phase II NHL-003 study (74). Patients received lenalidomide 25 mg days 1–21 of a 28-day cycle until disease progression or unacceptable toxicity. A 35% ORR including 12% CR was seen among MCL patients by blinded central review with a median PFS of 8.8 months and 23.0-month DOR in responding patients (57). Grade 3/4 toxicity was primarily hematologic and included neutropenia (41%), thrombocytopenia (19%), and anemia (9%). Interruptions or dose reductions were required in slightly over 50% of patients with neutropenia or thrombocytopenia the most common reason. Common non-hematologic toxicities included gastrointestinal symptoms, rash, and fatigue. In the phase II MCL-001 (EMERGE) trial, 134 patients with relapsed/refractory MCL previously treated with bortezomib were treated with lenalidomide (75). The ORR was 28% (8% CR), median PFS was 4.0 months, and DOR was 16.6 months (76). In the international phase II MCL-002 trial, 254 patients with relapsed/refractory MCL were randomized to either lenalidomide or investigator’s choice of therapy. PFS was 8.7 months with lenalidomide 25 mg versus 5.2 months with investigators choice which included single agent rituximab, fludarabine, gemcitabine, and chlorambucil (58). The addition of rituximab 375 mg/m2 weekly for four doses to lenalidomide 20 mg days 1-21 was studied in a phase II study of 52 patients with relapsed/refractory MCL with a 57% ORR (36% CR) and median PFS of 11.1 months (77). Hematologic toxicities included grade 3/4 neutropenia in 56% of patients and thrombocytopenia in 31%.
Temsirolimus (Torisel, Pfizer; New York City, NY, USA) is a prodrug of sirolimus, an inhibitor of the mammalian target of rapamycin (mTOR). Sirolimus has been shown to mediate decreased production and increased destruction of cyclin D1 (78), providing a rationale for mTOR inhibition in MCL. A phase II trial in relapsed/refractory MCL with temsirolimus 250 mg IV weekly achieved a 38% ORR and 3% CR rate, but 71% of patients experienced ≥ grade 3 hematological toxicities, and 9% experienced grade 4 toxicity (79). In a subsequent phase II study of temsirolimus 25 mg IV weekly, a 41% ORR was noted including one patient with CR, with lower rates of grade 3 toxicity including thrombocytopenia (39%) and neutropenia (18%) (80). In a phase III study, 162 patients with relapsed/refractory MCL were randomized to investigators choice of single agent therapy (most commonly gemcitabine or fludarabine) or temsirolimus dosed at 175 mg IV weekly for 3 weeks followed by 25 mg (175/25) weekly or 75 mg (175/75) weekly (61). The median PFS was 4.8 months with temsirolimus 175/75, 3.4 months with 175/25, and 1.9 months for investigator’s choice. While the benefit in PFS between 175/75 and investigators reached statistical significance, the ORR was only 22% for temsirolimus 175/75 (2% CR) and 2% for investigator’s choice. Non-hematologic adverse events (AE) with temsirolimus included diarrhea (44%), fever (39%), mucositis (35%), pruritis (26%), and edema (22%). Grade 3 or 4 toxicities included thrombocytopenia (59%), neutropenia (15%), rash (7%), and infection (9%). A subsequent phase III study was performed comparing temsirolimus dosed at 175/75 to ibrutinib in relapsed or refractory MCL (56). The ORR with temsirolimus was 40% including 1% CR, median PFS was 6.2 months, and AEs led to discontinuation of therapy in 26% of patients, including pneumonia or pneumonitis seen in 5 patients respectively. Temsirolimus was approved by the European Medicines Agency in 2006 for relapsed/refractory MCL, but does not have US FDA approval for this indication. With significant rates of discontinuation due to toxicity and lack of prolonged PFS in responding patients, temsirolimus has at best a very limited role in management of relapsed/refractory MCL and is inferior to ibrutinib.
Authors’ approach to treatment
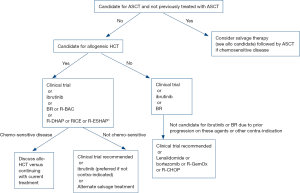
The author’s approach to management of patients with relapsed MCL is outlined in Figure 1. There is no standard treatment for relapsed MCL and participation in a well-designed clinical trial is our recommendation for patients with relapsed disease who are eligible. For patients who are ineligible for a clinical trial or who do not wish to participate, ibrutinib is our preferred treatment for many patients given the high response rates, tolerability, and median PFS of greater than 12 months. The majority of patients will not achieve a CR with ibrutinib, and combination chemo-immunotherapy may be required for transplant eligible patients with multiply relapsed disease for disease control prior to allogeneic HCT. BR is another generally well tolerated therapy with high response rates and median PFS greater than 1 year that is attractive for patients with relapsed disease who prefer to avoid indefinite daily oral therapy.
Future directions
Targeting Bcl-2 with venetoclax
B cell leukemia/lymphoma 2 (Bcl-2) is an anti-apoptotic protein key to the pathogenesis of multiple sub-types of NHL. The gene encoding Bcl2 resides on chromosome 18q21-22 (81,82). Copy number gains or amplification of 18q21-22 are frequently identified in MCL (83) and preclinical cell line models demonstrated activity of the small molecule Bcl-2 specific inhibitor venetoclax (Venclexta, formerly ABT-199, Abbvie Inc., North Chicago, IL, USA) versus MCL (84). In the NHL cohort of the M12-175 study, 106 patients with relapsed/refractory NHL including MCL were treated with oral venetoclax (85). Tumor lysis syndrome (TLS) occurred in earlier studies of venetoclax (86), and patients with bulky MCL (>10 cm) were hospitalized at initial treatment for monitoring, hydration, and supportive treatment (85). Grade 3 or 4 toxicities were hematologic; serious AEs included hyponatremia and febrile neutropenia. The most common AEs were nausea, diarrhea, fatigue, and headache. TLS was reported in 3 patients with bulky MCL within 24 hours of start of therapy and was managed supportively. Of the 24 MCL patients, ORR was 75% including 21% CR, with a median PFS of 14 months (85). None of the enrolled patients had previously received ibrutinib.
Venetoclax and ibrutinib synergistically induce apoptosis in MCL cell lines, leading to interest in combination therapy (87). Preliminary results were recently presented from the phase II AIM study (NCT02471391) of ibrutinib in combination with venetoclax (88). At 16 weeks, the ORR was 71% with 63% CR. Common AE included fatigue, diarrhea, nausea, neutropenia, bruising, and TLS. While mature results are needed before drawing conclusions regarding efficacy and longer follow up for DOR, the high observed CR rate is promising and compares favorably to historical trials of alternate therapies in the relapsed/refractory setting. A phase III study of ibrutinib combined with venetoclax versus placebo (SYMPATICO NCT03112174) is planned to determine whether combination therapy with ibrutinib and venetoclax is superior to ibrutinib monotherapy.
Type II CD-20 targeted antibody obinutuzumab
Obinutuzumab (Gazyva, formerly GA-101, Roche, Basel, Switzerland) is a type II glycoengineered monoclonal antibody with a distinct CD-20 binding orientation leading to increased antibody dependent cell-mediated cytotoxicity when compared with rituximab (89,90). In the phase II GAUDIN study, patients with relapsed/refractory MCL including patients with rituximab refractory disease were treated with obinutuzumab at two dosing levels (91). Of the 15 patients with MCL, 11 were treated at the lower dose level with 2 patients achieving CR, while 2 of 4 patients treated at the higher dose level (1,600 mg cycle 1 and 800 mg cycle 2) achieved CR. Toxicities included infusion reactions, TLS and thrombocytopenia. Obinutuzumab is currently being studied in combination with ibrutinib (NCT02736617), ibrutinib and venetoclax (NCT02558816), and in combination with entospletinib (NCT03010358) in patients with relapsed/refractory MCL.
Alternate BTK inhibitors
The efficacy of ibrutinib is thought to be due primarily to BTK inhibition, but ibrutinib has off target effects that contribute to toxicities including risk of atrial fibrillation and bleeding. Alternate selective BTK inhibitors offer potentially improved toxicity profile with less off target effects.
Acalabrutinib (formerly ACP-196, Acerta Pharma, Redwood City, CA, USA) is a second-generation irreversible BTK inhibitor with improved selectivity for BTK (92). In a phase I/II trial in CLL, acalabrutinib was well tolerated with the most common AEs headache, diarrhea, and weight gain with no reported cases of atrial fibrillation or major bleeding (93). A phase II study of acalabrutinib for the treatment of MCL has completed enrollment with preliminary results expected shortly (NCT02213926). Combination studies including acalabrutinib are currently underway in relapsed/refractory MCL including with BR (NCT02717624 and NCT02972840), the immune checkpoint inhibitor pembrolizumab (NCT02362035) and the PI3K inhibitor ACP-319 (NCT02328014).
Tirabrutinib (formerly GS-4059, Gilead Sciences, Foster City, CA, USA) is an alternate second-generation irreversible BTK inhibitor. In a phase I study including 16 MCL patients, grade 3 or higher toxicities were primarily hematologic and rash (94). One grade 3 bleeding event was observed. Gastrointestinal toxicity was reported less frequently than with alternate BTK inhibitors. Of 12 MCL patients evaluable, responses were noted in 11 patients including 5 patients with CR. In addition to an ongoing study of single agent tirabrutinib in patients with relapsed/refractory B-cell malignancies (NCT02457559), study of combination therapy with other targeted agents including idelalisib, obinutuzumab, and entospletinib is currently underway (NCT02457598).
Finally, BGB-3111 (BeiGene, Fort Lee, NJ, USA) is a selective, small molecule, irreversible BTK inhibitor currently under development for the treatment of patients with lymphoid malignancy. A phase I dose escalation study treated 6 MCL patients with escalating doses of BGB-3111 (95). Five patients achieved an objective response including 1 patient with CR. BGB-3111 is currently being studied in combination with obinutuzumab (NCT02569476) as well as in combination with the immune checkpoint inhibitor BGB-A317 (NCT02795182) in patients with relapsed/refractory lymphoid malignancy.
Targeting PI3K
Phosphatidylinositol-3-kinase (PI3K) is a lipid kinase critical to downstream BCR signaling leading to phosphorylation of AKT (96). In vitro inhibition of AKT and mTOR in MCL leads to degradation of cyclin D1, and inhibition of AKT leads to MCL cell apoptosis (97). PI3K consists of a regulatory and catalytic subunit with four catalytic isoforms, α, β, γ, and δ. PI3Kδ is essential to B-cell signaling (98), and inhibiting PI3Kδ blocks AKT phosphorylation in lymphoid malignancies (99). The PI3Kα isoform is variably present in MCL with greater expression following multiple lines of treatment, and increased expression of PI3Kα confers resistance to selective PI3Kδ inhibition in vitro which is overcome with combined PI3K α and δ inhibitors (100).
Idelalisib (Zydelig, formerly GS-1101 and CAL-101, Gilead Sciences, Foster City, CA) is a selective PI3Kδ inhibitor. In a phase I study, 40 patients with relapsed/refractory MCL were treated with idelalisib once or twice daily at doses ranging from 50 to 350 mg (101). Grade 3 or higher hematologic toxicities included neutropenia, anemia, and thrombocytopenia. Grade 3 or higher non-hematologic toxicities included elevated AST/ALT, diarrhea, anorexia and pneumonia. The ORR was 40% including 5% CR, but responses were not durable in the majority of patients with a median DOR of 2.7 months, median PFS of 3.7 months, and 1 year PFS of 22%. Given the activity with idelalisib and limited hematologic toxicity, combination therapy has been explored; however, a phase 1 study of idelalisib with rituximab and lenalidomide in patients with relapsed MCL (NCT 01838434) was halted due to unexpected toxicity (102) as was a study of idelalisib and entospletinib (103). A phase Ib study of idelalisib combined with the Bcl-2 inhibitor BCL-201 (NCT02603445) in patients with relapsed/refractory MCL is currently underway as is a study of idelalisib and tirabrutinib (NCT02457598).
Umbralisib (formerly TGR-1202 and RP-5264, TG Therapeutics, New York, NY, USA) is an alternative selective PI3Kδ inhibitor currently under investigation in lymphoid malignancies including an ongoing phase I/Ib study in combination with ibrutinib for the treatment of MCL (NCT 02268851). Preliminary results showed 11 of 13 evaluable MCL patients exhibiting objective response (ORR 85%, 8% CR) with reported ≥ grade 3 toxicities including neutropenia, lipase elevation, hypophosphatemia, atrial fibrillation, and infectious complications including CNS aspergillus infection in two patients (104). The recommended phase II dosage was ibrutinib 560 mg and umbralisib 800 mg daily.
Copanlisib (formerly BAY 80-6946; Bayer AG, Berlin, Germany) is an intravenous class IA PI3K α and δ inhibitor (105). In a phase II study, 33 patients with indolent lymphoma and 51 with aggressive lymphoma including MCL were treated with copanlisib 0.8 mg/kg on days 1, 8, and 15 of a 28-day cycle (106). Grade 3 or higher AE included lung infection, diarrhea, febrile neutropenia, hyperglycemia, and pancreatitis. Opportunistic infections included a fatal case of Cryptococcus neoformans meningitis and one case of Pneumocystis jirovecii pneumonitis. There were no reported episodes of colitis and elevation in liver function tests was not dose limiting suggesting a distinct toxicity profile in comparison to oral PI3Kδ inhibitors. The ORR was 64% in MCL patients including 2 of 11 patients with unconfirmed CR.
Targeting cyclin dependent kinase
MCL constitutively overexpresses cyclin D1 which complexes with cyclin dependent kinase (CDK) 4 leading to phosphorylation of retinoblastoma protein driving tumor proliferation (107). The pan-CDK inhibitor flavopiridol demonstrated clinical activity in a subset of patients with MCL supporting a role for the therapeutic application of CDK inhibition (108,109). Orally administered CDK 4/6 specific inhibitors offer strong CDK 4 and 6 inhibition as well as significantly less off target effect (110). The CDK 4/6 inhibitor palbociclib (Ibrance, formerly PD0332091, Pfizer, New York City, NY) was studied in a phase Ib pilot study in which 17 relapsed/refractory MCL patients were treated with oral palbociclib 125 mg daily for 3 out of 4 weeks of a 28 day cycle (111). AEs included neutropenia, thrombocytopenia, and diarrhea. While preclinical models of CDK 4 inhibition in MCL predicted arrest of cell growth rather than cell killing (112), objective response was seen in 3 patients (ORR 18%) including one CR (111). The median PFS was 4 months; responding patients experienced a DOR of 18 months or greater. An alternative CDK 4/6 inhibitor, abemaciclib (formerly LY2835219, Eli Lilly, Indianapolis, IN), was studied in 22 patients with relapsed/refractory MCL dosed at 200 mg every 12 hours (113). Hematologic toxicities included thrombocytopenia, neutropenia, and other toxicities included diarrhea, vomiting and fatigue. Five of 22 patients achieved PR (23%) and an additional 9 patients achieved stable disease. After 6 cycles (28 days per cycle), 8 patients remained on therapy with no evidence of disease progression.
Combination therapy with CDK 4/6 inhibitors is currently being explored. A Phase I study of ibrutinib and palbociclib in MCL has been completed with acceptable safety profile and an ORR of 67% including 44% CR which compares favorably to studies of single agent ibrutinib (114). A phase II study is planned. A phase I dose escalation study of palbociclib and bortezomib in relapsed/refractory MCL established palbociclib 125 mg on days 1–12 and bortezomib 1 mg/m2 twice weekly for 21-day cycles as the recommended phase II dose with higher dosage of bortezomib associated with prohibitive hematologic toxicity (115). Of the 7 patients treated at the recommended dose level, 4 experienced freedom from disease progression lasting greater than 12 months including 1 patient with CR.
Combination therapy with ibrutinib
Ibrutinib has a high single agent response rate but the majority of patients will not achieve CR and will ultimately develop resistance. This has led to interest in combination studies to improve depth and DOR. The combination of ibrutinib with rituximab was shown to be feasible with promising efficacy in a single center phase II trial enrolling 50 patients with relapsed/refractory MCL previously treated with rituximab containing regimens (116). Rituximab was administered weekly for four doses and then given on day 1 of cycles 3–8 with ibrutinib 560 mg continued daily throughout treatment. An 88% ORR was reported including 44% CR with 12 month PFS of 75%. Toxicities included diarrhea, sensory neuropathy, atrial fibrillation, and ≥ grade 3 bleeding. A Ki67 index of ≥50% was observed to be associated with lower response rate. While response rates with combination therapy compare favorably to those reported with single agent ibrutinib, comparison is limited due to the low percentage (12%) of patients with high risk MIPI at time of diagnosis.
The combination of BTK and proteasome inhibitors is currently being studied with trials of ibrutinib and bortezomib (NCT02356458) and ibrutinib and carfilzomib (NCT02269085) ongoing. The combination of lenalidomide and ibrutinib is being investigated in ongoing trials with and without rituximab (NCT02460276, NCT02446236, and NCT01955499). Preliminary results from the phase II MCL06 trial (PHILEMON) showed an 83% ORR including 41% CR, with median PFS not reached at 7 months and 7/13 patients achieving MRD negativity on bone marrow assessment following treatment with ibrutinib, lenalidomide, and rituximab (117). Multiple other ibrutinib containing combinations are currently under investigation with ongoing studies of targeted treatments for MCL summarized in Table 3.
Table 3
| Target | Drug | Combination | Trial |
|---|---|---|---|
| Bcl-2 | Venetoclax | + ibrutinib | NCT03112174 |
| CDK | Palbociclib | + ibrutinib | NCT02159755 |
| Abemaciclib | n/a | NCT01739309 | |
| BTK | Acalabrutinib | Single agent | NCT02213926 |
| + pembrolizumab | NCT02362035 | ||
| Tirabrutinib | Single agent | NCT02457559 | |
| + obinutuzumab | NCT02457598 | ||
| BGB-3111 | + obinutuzumab | NCT02569476 | |
| + BGB A317 | NCT02795182 | ||
| CD-20 | Obinutuzumab | + ibrutinib | NCT02736617 |
| + venetoclax | NCT02558816 | ||
| Ublituximab | + ibrutinib | NCT02013128 | |
| + lenalidomide | NCT01744912 | ||
| Proteasome | Carfilzomib | + ibrutinib | NCT02269085 |
| PI3K | Idelalisib | + tirabrutinib | NCT02457598 |
| + BCL 201 | NCT02603445 | ||
| Umbralisib | + ibrutinib | NCT02268851 | |
| + ublituximab | NCT02006485 | ||
| Copanlisib | |||
| SYK | Entospletinib | + obinutuzumab | NCT03010358 |
| + vincristine | NCT02568683 | ||
| + tirabrutinib | NCT02457598 | ||
| CD-19 | Axicabtagene ciloleucel | Conditioning chemotherapy | NCT02601313 |
| Tisagenlecleucel | |||
| PD-1 | Pembrolizumab | + ibrutinib | NCT03153202, NCT02950220 |
| Nivolumab | + lenalidomide | NCT03015896 | |
| Androgen receptor | Enzalutamide | n/a | NCT 02489123 |
| SINE | Selinexor | + ibrutinib | NCT02303392 |
MCL, mantle cell lymphoma; Bcl-2, B cell leukemia/ lymphoma 2; CDK, cyclin dependent kinase; BTK, Bruton’s tyrosine kinase; NF-κB, nuclear factor kappa B; PI3K, phosphatidylinositol-3-kinase; SYK, spleen tyrosine kinase; PD-1, programmed death 1; SINE, selective inhibitor of nuclear export.
Targeting spleen tyrosine kinase
Spleen tyrosine kinase (Syk) is an intracellular tyrosine kinase involved in downstream BCR signaling where it complexes with PI3K and phospholipase Cγ2 and is activated in lymphoid malignancies including MCL (118,119). The oral Syk inhibitor fostamatinib was studied in patients with CLL and NHL including MCL with clinical activity in a minority of patients including an ORR of 11% (1 of 9 patients) in MCL (120). Entospletinib (formerly GS-9973, Gilead Sciences, Foster City, CA) is an alternative Syk inhibitor with less off target effects in comparison with fostamatinib (121). Entospletinib was studied in a phase II trial of 186 patients with CLL or NHL including MCL. Safety results from the entire cohort have been published, with the most common grade 3 or higher non-hematologic AE increased ALT, fatigue, dyspnea, and nausea and ≥ grade 3 hematologic toxicities including neutropenia, anemia, and thrombocytopenia (122). Efficacy has not yet been reported for the MCL patients (122). Future trials of combination therapy with entospletinib for NHL including MCL are planned.
Chimeric antigen receptor (CAR) T cell therapy
CAR T cells are an approach to adoptive immunotherapy whereby T cells are modified using viral vectors to express a receptor for a tumor surface antigen coupled with a stimulatory intracellular signaling domain. CAR-T cells targeting the B cell surface marker CD-19 have demonstrated encouraging results in patients with relapsed/refractory B cell NHL, with >50% CR rate in heavily pretreated patients with conditioning chemotherapy followed by autologous CD-19 targeted CAR-T cell infusion (123,124). The ZUMA-2 trial (NCT02601313) is currently underway assessing the safety and efficacy of conditioning chemotherapy with FC followed by infusion of the autologous CD-19 CAR-T construct axicabtagene ciloleucel (formerly KTE-C19, Kite Pharma, Los Angeles, CA, USA) in patients with relapsed/refractory MCL. Trials with alternate CAR-T constructs are ongoing assessing whether the safety and/or efficacy of CAR-T therapies can be improved with the addition of immunomodulatory medications including immune checkpoint inhibitors (NCT02926833) or ibrutinib (NCT02640209). Results from these and other studies are eagerly anticipated as the role of CAR-T therapy in the treatment of MCL remains to be seen.
Conclusions
Although the management of relapsed MCL is challenging due both to its aggressive clinical course and the limited prospective data specific to this relatively rare disease, significant progress has been made recently including the development of targeted therapies and the successful completion of phase III trials for relapsed and refractory disease. Advances in our understanding of disease biology are currently being translated into a growing list of targeted agents under clinical development. Expanding options for treatment appear to be on the horizon, and combinations of targeted therapies and advances in adoptive immunotherapy have the potential to shift the treatment paradigm of this disease in the years to come.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Bruce D. Cheson) for the series “Inaugural Issue” published in Annals of Lymphoma. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aol.2017.10.01). The series “Inaugural Issue” was commissioned by the editorial office without any funding or sponsorship. KAB serves as an unpaid editorial board member of Annals of Lymphoma from Jan 2017 to Jan 2019. KM reports that she is a consultant for Janssen, for BMS, and for Pharmacyclics, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Oka K, Ohno T, Kita K, et al. PRAD1 gene over-expression in mantle-cell lymphoma but not in other low-grade B-cell lymphomas, including extranodal lymphoma. Br J Haematol 1994;86:786-91. [Crossref] [PubMed]
- Ek S, Dictor M, Jerkeman M, et al. Nuclear expression of the non B-cell lineage Sox11 transcription factor identifies mantle cell lymphoma. Blood 2008;111:800-5. [Crossref] [PubMed]
- Salaverria I, Royo C, Carvajal-Cuenca A, et al. CCND2 rearrangements are the most frequent genetic events in cyclin D1(-) mantle cell lymphoma. Blood 2013;121:1394-402. [Crossref] [PubMed]
- Zucca E, Roggero E, Pinotti G, et al. Patterns of survival in mantle cell lymphoma. Ann Oncol 1995;6:257-62. [Crossref] [PubMed]
- Zhou Y, Wang H, Fang W, et al. Incidence trends of mantle cell lymphoma in the United States between 1992 and 2004. Cancer 2008;113:791-8. [Crossref] [PubMed]
- Argatoff LH, Connors JM, Klasa RJ, et al. Mantle cell lymphoma: a clinicopathologic study of 80 cases. Blood 1997;89:2067-78. [PubMed]
- Hoster E, Dreyling M, Klapper W, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood 2008;111:558-65. [Crossref] [PubMed]
- Herrmann A, Hoster E, Zwingers T, et al. Improvement of overall survival in advanced stage mantle cell lymphoma. J Clin Oncol 2009;27:511-8. [Crossref] [PubMed]
- Geisler CH, Kolstad A, Laurell A, et al. Nordic MCL2 trial update: six-year follow-up after intensive immunochemotherapy for untreated mantle cell lymphoma followed by BEAM or BEAC + autologous stem-cell support: still very long survival but late relapses do occur. Br J Haematol 2012;158:355-62. [Crossref] [PubMed]
- Fernàndez V, Salamero O, Espinet B, et al. Genomic and gene expression profiling defines indolent forms of mantle cell lymphoma. Cancer Res 2010;70:1408-18. [Crossref] [PubMed]
- Navarro A, Clot G, Royo C, et al. Molecular subsets of mantle cell lymphoma defined by the IGHV mutational status and SOX11 expression have distinct biologic and clinical features. Cancer Res 2012;72:5307-16. [Crossref] [PubMed]
- Royo C, Navarro A, Clot G, et al. Non-nodal type of mantle cell lymphoma is a specific biological and clinical subgroup of the disease. Leukemia 2012;26:1895-8. [Crossref] [PubMed]
- Cohen JB, Han X, Jemal A, et al. Deferred therapy is associated with improved overall survival in patients with newly diagnosed mantle cell lymphoma. Cancer 2016;122:2356-63. [Crossref] [PubMed]
- Martin P, Chadburn A, Christos P, et al. Outcome of deferred initial therapy in mantle-cell lymphoma. J Clin Oncol 2009;27:1209-13. [Crossref] [PubMed]
- Witzig TE, Geyer SM, Kurtin PJ, et al. Salvage chemotherapy with rituximab DHAP for relapsed non-Hodgkin lymphoma: a phase II trial in the North Central Cancer Treatment Group. Leuk Lymphoma 2008;49:1074-80. [Crossref] [PubMed]
- Velasquez WS, McLaughlin P, Tucker S, et al. ESHAP--an effective chemotherapy regimen in refractory and relapsing lymphoma: a 4-year follow-up study. J Clin Oncol 1994;12:1169-76. [Crossref] [PubMed]
- Visco C, Finotto S, Zambello R, et al. Combination of rituximab, bendamustine, and cytarabine for patients with mantle-cell non-Hodgkin lymphoma ineligible for intensive regimens or autologous transplantation. J Clin Oncol 2013;31:1442-9. [Crossref] [PubMed]
- Harting R, Venugopal P, Gregory SA, et al. Efficacy and safety of rituximab combined with ESHAP chemotherapy for the treatment of relapsed/refractory aggressive B-cell non-Hodgkin lymphoma. Clin Lymphoma Myeloma 2007;7:406-12. [Crossref] [PubMed]
- Rummel MJ, Al-Batran SE, Kim SZ, et al. Bendamustine plus rituximab is effective and has a favorable toxicity profile in the treatment of mantle cell and low-grade non-Hodgkin's lymphoma. J Clin Oncol 2005;23:3383-9. [Crossref] [PubMed]
- Stewart DA, Duan Q, Carlson L, et al. A prospective phase II study of RICE re-induction, then high-dose fludarabine and busulfan, followed by autologous or allogeneic blood stem cell transplantation for indolent b-cell lymphoma. Clin Lymphoma Myeloma Leuk 2011;11:475-82. [Crossref] [PubMed]
- Moskowitz CH, Bertino JR, Glassman JR, et al. Ifosfamide, carboplatin, and etoposide: a highly effective cytoreduction and peripheral-blood progenitor-cell mobilization regimen for transplant-eligible patients with non-Hodgkin's lymphoma. J Clin Oncol 1999;17:3776-85. [Crossref] [PubMed]
- Vandenberghe E, Ruiz de Elvira C, Loberiza FR, et al. Outcome of autologous transplantation for mantle cell lymphoma: a study by the European Blood and Bone Marrow Transplant and Autologous Blood and Marrow Transplant Registries. Br J Haematol 2003;120:793-800. [Crossref] [PubMed]
- Till BG, Gooley TA, Crawford N, et al. Effect of remission status and induction chemotherapy regimen on outcome of autologous stem cell transplantation for mantle cell lymphoma. Leuk Lymphoma 2008;49:1062-73. [Crossref] [PubMed]
- Fenske TS, Zhang MJ, Carreras J, et al. Autologous or reduced-intensity conditioning allogeneic hematopoietic cell transplantation for chemotherapy-sensitive mantle-cell lymphoma: analysis of transplantation timing and modality. J Clin Oncol 2014;32:273-81. [Crossref] [PubMed]
- Cassaday RD, Guthrie KA, Budde EL, et al. Specific features identify patients with relapsed or refractory mantle cell lymphoma benefitting from autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant 2013;19:1403-6. [Crossref] [PubMed]
- Dietrich S, Boumendil A, Finel H, et al. Outcome and prognostic factors in patients with mantle-cell lymphoma relapsing after autologous stem-cell transplantation: a retrospective study of the European Group for Blood and Marrow Transplantation (EBMT). Ann Oncol 2014;25:1053-8. [Crossref] [PubMed]
- Vaughn JE, Sorror ML, Storer BE, et al. Long-term sustained disease control in patients with mantle cell lymphoma with or without active disease after treatment with allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Cancer 2015;121:3709-16. [Crossref] [PubMed]
- Tessoulin B, Ceballos P, Chevallier P, et al. Allogeneic stem cell transplantation for patients with mantle cell lymphoma who failed autologous stem cell transplantation: a national survey of the SFGM-TC. Bone Marrow Transplant 2016;51:1184-90. [Crossref] [PubMed]
- Khouri IF, Lee MS, Saliba RM, et al. Nonablative allogeneic stem-cell transplantation for advanced/recurrent mantle-cell lymphoma. J Clin Oncol 2003;21:4407-12. [Crossref] [PubMed]
- Krüger WH, Hirt C, Basara N, et al. Allogeneic stem cell transplantation for mantle cell lymphoma--final report from the prospective trials of the East German Study Group Haematology/Oncology (OSHO). Ann Hematol 2014;93:1587-97. [Crossref] [PubMed]
- Tam CS, Bassett R, Ledesma C, et al. Mature results of the M. D. Anderson Cancer Center risk-adapted transplantation strategy in mantle cell lymphoma. Blood 2009;113:4144-52. [Crossref] [PubMed]
- Cook G, Smith GM, Kirkland K, et al. Outcome following Reduced-Intensity Allogeneic Stem Cell Transplantation (RIC AlloSCT) for relapsed and refractory mantle cell lymphoma (MCL): a study of the British Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant 2010;16:1419-27. [Crossref] [PubMed]
- Kharfan-Dabaja MA, Reljic T, El-Asmar J, et al. Reduced-intensity or myeloablative allogeneic hematopoietic cell transplantation for mantle cell lymphoma: a systematic review. Future Oncol 2016;12:2631-42. [Crossref] [PubMed]
- Cruz JG, Martino R, Balsalobre P, et al. Long-Term Results of Fludarabine/Melphalan as a Reduced-Intensity Conditioning Regimen in Mantle Cell Lymphoma: The GELTAMO Experience. Ther Adv Hematol 2011;2:5-10. [Crossref] [PubMed]
- Ganti AK, Bierman PJ, Lynch JC, et al. Hematopoietic stem cell transplantation in mantle cell lymphoma. Ann Oncol 2005;16:618-24. [Crossref] [PubMed]
- Le Gouill S, Kroger N, Dhedin N, et al. Reduced-intensity conditioning allogeneic stem cell transplantation for relapsed/refractory mantle cell lymphoma: a multicenter experience. Ann Oncol 2012;23:2695-703. [Crossref] [PubMed]
- Hamadani M, Benson DM Jr, Hofmeister CC, et al. Allogeneic stem cell transplantation for patients with relapsed chemorefractory aggressive non-hodgkin lymphomas. Biol Blood Marrow Transplant 2009;15:547-53. [Crossref] [PubMed]
- Hamadani M, Saber W, Ahn KW, et al. Allogeneic hematopoietic cell transplantation for chemotherapy-unresponsive mantle cell lymphoma: a cohort analysis from the center for international blood and marrow transplant research. Biol Blood Marrow Transplant 2013;19:625-31. [Crossref] [PubMed]
- Robinson KS, Williams ME, van der Jagt RH, et al. Phase II multicenter study of bendamustine plus rituximab in patients with relapsed indolent B-cell and mantle cell non-Hodgkin's lymphoma. J Clin Oncol 2008;26:4473-9. [Crossref] [PubMed]
- Rummel M, Kaiser U, Balser C, et al. Bendamustine plus rituximab versus fludarabine plus rituximab for patients with relapsed indolent and mantle-cell lymphomas: a multicentre, randomised, open-label, non-inferiority phase 3 trial. Lancet Oncol 2016;17:57-66. [Crossref] [PubMed]
- Hitz F, Martinelli G, Zucca E, et al. A multicentre phase II trial of gemcitabine for the treatment of patients with newly diagnosed, relapsed or chemotherapy resistant mantle cell lymphoma: SAKK 36/03. Hematol Oncol 2009;27:154-9. [Crossref] [PubMed]
- Morschhauser F, Depil S, Jourdan E, et al. Phase II study of gemcitabine-dexamethasone with or without cisplatin in relapsed or refractory mantle cell lymphoma. Ann Oncol 2007;18:370-5. [Crossref] [PubMed]
- Obrador-Hevia A, Serra-Sitjar M, Rodriguez J, et al. Efficacy of the GemOx-R regimen leads to the identification of Oxaliplatin as a highly effective drug against Mantle Cell Lymphoma. Br J Haematol 2016;174:899-910. [Crossref] [PubMed]
- Furtado M, Johnson R, Kruger A, et al. Addition of bortezomib to standard dose chop chemotherapy improves response and survival in relapsed mantle cell lymphoma. Br J Haematol 2015;168:55-62. [Crossref] [PubMed]
- Thomas DW, Owen RG, Johnson SA, et al. Superior quality and duration of responses among patients with mantle-cell lymphoma treated with fludarabine and cyclophosphamide with or without rituximab compared with prior responses to CHOP. Leuk Lymphoma 2005;46:549-52. [Crossref] [PubMed]
- Ohmachi K, Ando K, Ogura M, et al. Multicenter phase II study of bendamustine for relapsed or refractory indolent B-cell non-Hodgkin lymphoma and mantle cell lymphoma. Cancer Sci 2010;101:2059-64. [Crossref] [PubMed]
- Visco C, Chiappella A, Nassi L, et al. Rituximab, bendamustine, and low-dose cytarabine as induction therapy in elderly patients with mantle cell lymphoma: a multicentre, phase 2 trial from Fondazione Italiana Linfomi. Lancet Haematol 2017;4:e15-e23. [Crossref] [PubMed]
- Maddocks K, Christian B, Jaglowski S, et al. A phase 1/1b study of rituximab, bendamustine, and ibrutinib in patients with untreated and relapsed/refractory non-Hodgkin lymphoma. Blood 2015;125:242-8. [Crossref] [PubMed]
- Lenz G, Dreyling M, Hoster E, et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG). J Clin Oncol 2005;23:1984-92. [Crossref] [PubMed]
- Forstpointner R, Dreyling M, Repp R, et al. The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival as compared with FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood 2004;104:3064-71. [Crossref] [PubMed]
- Dubovsky JA, Beckwith KA, Natarajan G, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood 2013;122:2539-49. [Crossref] [PubMed]
- Advani RH, Buggy JJ, Sharman JP, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol 2013;31:88-94. [Crossref] [PubMed]
- Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med 2013;369:507-16. [Crossref] [PubMed]
- Wang ML, Blum KA, Martin P, et al. Long-term follow-up of MCL patients treated with single-agent ibrutinib: updated safety and efficacy results. Blood 2015;126:739-45. [Crossref] [PubMed]
- Wang M, Goy A, Martin P. Efficacy and Safety of Single-Agent Ibrutinib in Patients with Mantle Cell Lymphoma Who Progressed after Bortezomib Therapy. Blood 2014;124:4471.
- Dreyling M, Jurczak W, Jerkeman M, et al. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: an international, randomised, open-label, phase 3 study. Lancet 2016;387:770-8. [Crossref] [PubMed]
- Zinzani PL, Vose JM, Czuczman MS, et al. Long-term follow-up of lenalidomide in relapsed/refractory mantle cell lymphoma: subset analysis of the NHL-003 study. Ann Oncol 2013;24:2892-7. [Crossref] [PubMed]
- Trneny M, Lamy T, Walewski J, et al. Lenalidomide versus investigator's choice in relapsed or refractory mantle cell lymphoma (MCL-002; SPRINT): a phase 2, randomised, multicentre trial. Lancet Oncol 2016;17:319-31. [Crossref] [PubMed]
- Goy A, Younes A, McLaughlin P, et al. Phase II study of proteasome inhibitor bortezomib in relapsed or refractory B-cell non-Hodgkin's lymphoma. J Clin Oncol 2005;23:667-75. [Crossref] [PubMed]
- Goy A, Bernstein SH, Kahl BS, et al. Bortezomib in patients with relapsed or refractory mantle cell lymphoma: updated time-to-event analyses of the multicenter phase 2 PINNACLE study. Ann Oncol 2009;20:520-5. [Crossref] [PubMed]
- Hess G, Herbrecht R, Romaguera J, et al. Phase III study to evaluate temsirolimus compared with investigator's choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J Clin Oncol 2009;27:3822-9. [Crossref] [PubMed]
- Cheah CY, Chihara D, Romaguera JE, et al. Patients with mantle cell lymphoma failing ibrutinib are unlikely to respond to salvage chemotherapy and have poor outcomes. Ann Oncol 2015;26:1175-9. [Crossref] [PubMed]
- Martin P, Maddocks K, Leonard JP, et al. Postibrutinib outcomes in patients with mantle cell lymphoma. Blood 2016;127:1559-63. [Crossref] [PubMed]
- Epperla N, Hamadani M, Cashen AF, et al. Predictive factors and outcomes for ibrutinib therapy in relapsed/refractory mantle cell lymphoma-a "real world" study. Hematol Oncol 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Chen W, Zeng D, Desai AP. Improved outcome for patients with relapsed/refractory mantle cell lymphoma (MCL) who stop ibrutinib +/− rituximab for reasons other than progression of disease. Hematol Oncol 2017;35:360. [Crossref]
- Srour SA, Lee HJ, Nomie K, et al. Novel chemotherapy-free combination regimen for ibrutinib-resistant mantle cell lymphoma. Br J Haematol 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Hideshima T, Mitsiades C, Akiyama M, et al. Molecular mechanisms mediating antimyeloma activity of proteasome inhibitor PS-341. Blood 2003;101:1530-4. [Crossref] [PubMed]
- Pham LV, Tamayo AT, Yoshimura LC, et al. Inhibition of constitutive NF-kappa B activation in mantle cell lymphoma B cells leads to induction of cell cycle arrest and apoptosis. J Immunol 2003;171:88-95. [Crossref] [PubMed]
- Fisher RI, Bernstein SH, Kahl BS, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol 2006;24:4867-74. [Crossref] [PubMed]
- Baiocchi RA, Alinari L, Lustberg ME, et al. Phase 2 trial of rituximab and bortezomib in patients with relapsed or refractory mantle cell and follicular lymphoma. Cancer 2011;117:2442-51. [Crossref] [PubMed]
- Lamm W, Kaufmann H, Raderer M, et al. Bortezomib combined with rituximab and dexamethasone is an active regimen for patients with relapsed and chemotherapy-refractory mantle cell lymphoma. Haematologica 2011;96:1008-14. [Crossref] [PubMed]
- Friedberg JW, Vose JM, Kelly JL, et al. The combination of bendamustine, bortezomib, and rituximab for patients with relapsed/refractory indolent and mantle cell non-Hodgkin lymphoma. Blood 2011;117:2807-12. [Crossref] [PubMed]
- Lee HJ, Romaguera JE, Feng L, et al. Phase II Study of Bortezomib in Combination with Cyclophosphamide and Rituximab for Relapsed or Refractory Mantle Cell Lymphoma. Oncologist 2017;22:549-53. [Crossref] [PubMed]
- Habermann TM, Lossos IS, Justice G, et al. Lenalidomide oral monotherapy produces a high response rate in patients with relapsed or refractory mantle cell lymphoma. Br J Haematol 2009;145:344-9. [Crossref] [PubMed]
- Goy A, Sinha R, Williams ME, et al. Single-agent lenalidomide in patients with mantle-cell lymphoma who relapsed or progressed after or were refractory to bortezomib: phase II MCL-001 (EMERGE) study. J Clin Oncol 2013;31:3688-95. [Crossref] [PubMed]
- Goy A, Kalayoglu Besisik S, Drach J, et al. Longer-term follow-up and outcome by tumour cell proliferation rate (Ki-67) in patients with relapsed/refractory mantle cell lymphoma treated with lenalidomide on MCL-001(EMERGE) pivotal trial. Br J Haematol 2015;170:496-503. [Crossref] [PubMed]
- Wang M, Fayad L, Wagner-Bartak N, et al. Lenalidomide in combination with rituximab for patients with relapsed or refractory mantle-cell lymphoma: a phase 1/2 clinical trial. Lancet Oncol 2012;13:716-23. [Crossref] [PubMed]
- Hashemolhosseini S, Nagamine Y, Morley SJ, et al. Rapamycin inhibition of the G1 to S transition is mediated by effects on cyclin D1 mRNA and protein stability. J Biol Chem 1998;273:14424-9. [Crossref] [PubMed]
- Witzig TE, Geyer SM, Ghobrial I, et al. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol 2005;23:5347-56. [Crossref] [PubMed]
- Ansell SM, Inwards DJ, Rowland KM Jr, et al. Low-dose, single-agent temsirolimus for relapsed mantle cell lymphoma: a phase 2 trial in the North Central Cancer Treatment Group. Cancer 2008;113:508-14. [Crossref] [PubMed]
- Tsujimoto Y, Finger LR, Yunis J, et al. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science 1984;226:1097-9. [Crossref] [PubMed]
- Pegoraro L, Palumbo A, Erikson J, et al. A 14;18 and an 8;14 chromosome translocation in a cell line derived from an acute B-cell leukemia. Proc Natl Acad Sci U S A 1984;81:7166-70. [Crossref] [PubMed]
- Bentz M, Plesch A, Bullinger L, et al. t(11;14)-positive mantle cell lymphomas exhibit complex karyotypes and share similarities with B-cell chronic lymphocytic leukemia. Genes Chromosomes Cancer 2000;27:285-94. [Crossref] [PubMed]
- Souers AJ, Leverson JD, Boghaert ER, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med 2013;19:202-8. [Crossref] [PubMed]
- Davids MS, Roberts AW, Seymour JF, et al. Phase I First-in-Human Study of Venetoclax in Patients With Relapsed or Refractory Non-Hodgkin Lymphoma. J Clin Oncol 2017;35:826-33. [Crossref] [PubMed]
- Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med 2016;374:311-22. [Crossref] [PubMed]
- Portell C, Axelrod M, Brett L. Synergistic Cytotoxicity of Ibrutinib and the BCL2 Antagonist, ABT-199(GDC-0199) in Mantle Cell Lymphoma (MCL) and Chronic Lymphocytic Leukemia (CLL): Molecular Analysis Reveals Mechanisms of Target Interactions. Blood 2014;124:509.
- Tam CS, Roberts AW, Anderson MA. Combination Ibrutinib (IBR) and Venetoclax (VEN) for the Treatment of Mantle Cell Lymphoma (MCL): Primary Endpoint Assessment of the Phase 2 AIM Study. J Clin Oncol 2017;35:abstr 7520.
- Niederfellner G, Lammens A, Mundigl O, et al. Epitope characterization and crystal structure of GA101 provide insights into the molecular basis for type I/II distinction of CD20 antibodies. Blood 2011;118:358-67. [Crossref] [PubMed]
- Mössner E, Brunker P, Moser S, et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood 2010;115:4393-402. [Crossref] [PubMed]
- Morschhauser FA, Cartron G, Thieblemont C, et al. Obinutuzumab (GA101) monotherapy in relapsed/refractory diffuse large b-cell lymphoma or mantle-cell lymphoma: results from the phase II GAUGUIN study. J Clin Oncol 2013;31:2912-9. [Crossref] [PubMed]
- Herman SEM, Montraveta A, Niemann CU, et al. The Bruton Tyrosine Kinase (BTK) Inhibitor Acalabrutinib Demonstrates Potent On-Target Effects and Efficacy in Two Mouse Models of Chronic Lymphocytic Leukemia. Clin Cancer Res 2017;23:2831-41. [Crossref] [PubMed]
- Byrd JC, Harrington B, O'Brien S, et al. Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med 2016;374:323-32. [Crossref] [PubMed]
- Walter HS, Rule SA, Dyer MJ, et al. A phase 1 clinical trial of the selective BTK inhibitor ONO/GS-4059 in relapsed and refractory mature B-cell malignancies. Blood 2016;127:411-9. [Crossref] [PubMed]
- Tam C, Grigg A, Opat S. The BTK Inhibitor, Bgb-3111, Is Safe, Tolerable, and Highly Active in Patients with Relapsed/ Refractory B-Cell Malignancies: Initial Report of a Phase 1 First-in-Human Trial. Blood 2015;126:832.
- Srinivasan L, Sasaki Y, Calado DP, et al. PI3 kinase signals BCR-dependent mature B cell survival. Cell 2009;139:573-86. [Crossref] [PubMed]
- Dal Col J, Zancai P, Terrin L, et al. Distinct functional significance of Akt and mTOR constitutive activation in mantle cell lymphoma. Blood 2008;111:5142-51. [Crossref] [PubMed]
- Bilancio A, Okkenhaug K, Camps M, et al. Key role of the p110delta isoform of PI3K in B-cell antigen and IL-4 receptor signaling: comparative analysis of genetic and pharmacologic interference with p110delta function in B cells. Blood 2006;107:642-50. [Crossref] [PubMed]
- Lannutti BJ, Meadows SA, Herman SE, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood 2011;117:591-4. [Crossref] [PubMed]
- Iyengar S, Clear A, Bodor C, et al. P110alpha-mediated constitutive PI3K signaling limits the efficacy of p110delta-selective inhibition in mantle cell lymphoma, particularly with multiple relapse. Blood 2013;121:2274-84. [Crossref] [PubMed]
- Kahl BS, Spurgeon SE, Furman RR, et al. A phase 1 study of the PI3Kdelta inhibitor idelalisib in patients with relapsed/refractory mantle cell lymphoma (MCL). Blood 2014;123:3398-405. [Crossref] [PubMed]
- Smith SM, Pitcher BN, Jung SH, et al. Safety and tolerability of idelalisib, lenalidomide, and rituximab in relapsed and refractory lymphoma: the Alliance for Clinical Trials in Oncology A051201 and A051202 phase 1 trials. Lancet Haematol 2017;4:e176-e182. [Crossref] [PubMed]
- Barr PM, Saylors GB, Spurgeon SE, et al. Phase 2 study of idelalisib and entospletinib: pneumonitis limits combination therapy in relapsed refractory CLL and NHL. Blood 2016;127:2411-5. [Crossref] [PubMed]
- Davids MS, Kim H, Nicotra A. Updated Results of a Multicenter Phase I/Ib Study of TGR-1202 in Combination with Ibrutinib in Patients with Relapsed or Refractory MCL or CLL. Hematol Oncol 2017;35:54-9. [Crossref] [PubMed]
- Liu N, Rowley BR, Bull CO, et al. BAY 80-6946 is a highly selective intravenous PI3K inhibitor with potent p110alpha and p110delta activities in tumor cell lines and xenograft models. Mol Cancer Ther 2013;12:2319-30. [Crossref] [PubMed]
- Dreyling M, Morschhauser F, Bouabdallah K, et al. Phase II study of copanlisib, a PI3K inhibitor, in relapsed or refractory, indolent or aggressive lymphoma. Ann Oncol 2017;28:2169-78. [Crossref] [PubMed]
- Sellers WR, Kaelin WG Jr. Role of the retinoblastoma protein in the pathogenesis of human cancer. J Clin Oncol 1997;15:3301-12. [Crossref] [PubMed]
- Jones JA, Rupert AS, Poi M, et al. Flavopiridol can be safely administered using a pharmacologically derived schedule and demonstrates activity in relapsed and refractory non-Hodgkin's lymphoma. Am J Hematol 2014;89:19-24. [Crossref] [PubMed]
- Lin TS, Blum KA, Fischer DB, et al. Flavopiridol, fludarabine, and rituximab in mantle cell lymphoma and indolent B-cell lymphoproliferative disorders. J Clin Oncol 2010;28:418-23. [Crossref] [PubMed]
- Toogood PL, Harvey PJ, Repine JT, et al. Discovery of a potent and selective inhibitor of cyclin-dependent kinase 4/6. J Med Chem 2005;48:2388-406. [Crossref] [PubMed]
- Leonard JP, LaCasce AS, Smith MR, et al. Selective CDK4/6 inhibition with tumor responses by PD0332991 in patients with mantle cell lymphoma. Blood 2012;119:4597-607. [Crossref] [PubMed]
- Marzec M, Kasprzycka M, Lai R, et al. Mantle cell lymphoma cells express predominantly cyclin D1a isoform and are highly sensitive to selective inhibition of CDK4 kinase activity. Blood 2006;108:1744-50. [Crossref] [PubMed]
- Morschhauser F, Bouabdallah K, Stilgenbauer S. Clinical Activity of Abemaciclib (LY2835219), a Cell Cycle Inhibitor Selective for CDK4 and CDK6, in Patients with Relapsed or Refractory Mantle Cell Lymphoma. Blood 2014;124:3067.
- Martin P, Blum K, Bartlett N. A Phase I Trial of Ibrutinib Plus Palbociclib in Patients with Previously Treated Mantle Cell Lymphoma. Blood 2016;128:150. [PubMed]
- Martin P, DiLiberto M, Mason CE. The Combination Of Palbociclib Plus Bortezomib Is Safe and Active In Patients With Previously Treated Mantle Cell Lymphoma: Final Results Of a Phase I Trial. Blood 2013;122:4393.
- Wang ML, Lee H, Chuang H, et al. Ibrutinib in combination with rituximab in relapsed or refractory mantle cell lymphoma: a single-centre, open-label, phase 2 trial. Lancet Oncol 2016;17:48-56. [Crossref] [PubMed]
- Jerkeman M, Hutchings M, Raty R. Ibrutinib-Lenalidomide-Rituximab in Patients with Relapsed/Refractory Mantle Cell Lymphoma: First Results from the Nordic Lymphoma Group MCL6 (PHILEMON) Phase II Trial. Blood 2016;128:148.
- Gobessi S, Laurenti L, Longo PG, et al. Inhibition of constitutive and BCR-induced Syk activation downregulates Mcl-1 and induces apoptosis in chronic lymphocytic leukemia B cells. Leukemia 2009;23:686-97. [Crossref] [PubMed]
- Rinaldi A, Kwee I, Taborelli M, et al. Genomic and expression profiling identifies the B-cell associated tyrosine kinase Syk as a possible therapeutic target in mantle cell lymphoma. Br J Haematol 2006;132:303-16. [Crossref] [PubMed]
- Friedberg JW, Sharman J, Sweetenham J, et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood 2010;115:2578-85. [Crossref] [PubMed]
- Currie KS, Kropf JE, Lee T, et al. Discovery of GS-9973, a selective and orally efficacious inhibitor of spleen tyrosine kinase. J Med Chem 2014;57:3856-73. [Crossref] [PubMed]
- Sharman J, Hawkins M, Kolibaba K, et al. An open-label phase 2 trial of entospletinib (GS-9973), a selective spleen tyrosine kinase inhibitor, in chronic lymphocytic leukemia. Blood 2015;125:2336-43. [Crossref] [PubMed]
- Kochenderfer JN, Somerville RP, Lu T, et al. Lymphoma Remissions Caused by Anti-CD19 Chimeric Antigen Receptor T Cells Are Associated With High Serum Interleukin-15 Levels. J Clin Oncol 2017;35:1803-13. [Crossref] [PubMed]
- Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol 2015;33:540-9. [Crossref] [PubMed]
Cite this article as: Bond DA, Blum KA, Maddocks K. Management of relapsed and refractory mantle cell lymphoma: a review of current evidence and future directions for research. Ann Lymphoma 2017;1:5.


