
Is reimplant after breast implant-associated anaplastic large cell lymphoma a safe procedure? Yes, no, or may be?
Introduction
Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) is a rare type of T-cell non-Hodgkin lymphoma, classified as a provisional entity in the 2016 WHO classification of lymphoid neoplasms (1). The disease has morphological and immunophenotypic features indistinguishable from those of ALK-negative ALCL, arising primarily in association with a breast implant.
The first case of BIA-ALCL was described in 1997 by Keech et al., as a lymphoma arising in proximity to a saline-filled breast implant (2). Usually, it occurs in fluid collected in an implant surrounding after surgery for aesthetic or reconstructive reason.
The real incidence of BIA-ALCL still remains unknown, although the risk of developing a BIA-ALCL is estimated in one case per 500,000 women with breast implants (1,3,4)
Since the first report, approximately 400 cases of this rare entity have been reported in the literature so far (3,5). Due to large number of breast augmentation procedures and reconstructions after mastectomy, it is reasonable estimating that an increasing number of women are at risk for developing BIA-ALCL.
BIA-ALCL usually occurs 3–14 years after aesthetic or reconstructive breast augmentation, with different types of implants (saline or silicone) (6,7). Frequently, patients present with seroma or fluid effusion in implant surrounding, breast inflammation, swelling and asymmetry.
The issues on etiology and pathogenesis are still controversial and matter of debate. However, there is increasing evidence that inflammatory reaction to chronic bacterial/biofilm infection and use of textured implants may promote the development of BIA-ALCL (7,8).
The prognosis of this type of lymphoma is excellent, once the breast implants with the capsule are removed. Current treatment strategies indicate that the removal of both breast implants is required, because a small number of cases that have been diagnosed on both sides at the same time. However, a small group of patients have a tumor mass and may require additional therapy.
While exhaustive guidelines on treatment of BIA-ALCL are already available (9) there is no data available on the safety, and timing, of new breast implant insertion for aesthetic and psychological aspects, once BIA-ALCL has been successfully treated.
Here we like to share with the readers the case of a 48 years old woman diagnosed with stage I BIA-ALCL developed 8 years after a textured, silicone filled breast implant was performed for aesthetic reasons, and treated with surgical removal six months earlier. She came to us with the following intriguing questions: “May I undergo a new breast implant insertion? Which implant type should I consider? And, when the new implant could be re-inserted?”.
Case presentation
A 48 years old lady presented at our attention on November 2017, with a recent diagnosis of BIA-ALCL, developed eight years after implantation with breast silicone and textured implant for cosmetic reason in 2009.
Since February, 2017 patient was complaining with swelling associated with skin rash surrounding the right reconstructed breast. An MRI evaluation was performed detecting a peri-protestethic fluid collection (Figure 1). Other symptoms such as mass, enlargement of lymph nodes or capsular disruption were not reported. The patient underwent an ultrasound guided aspiration of pericapsular fluid. The cytologic analysis revealed the presence of atypical medium-to-large cells with high CD30 expression.
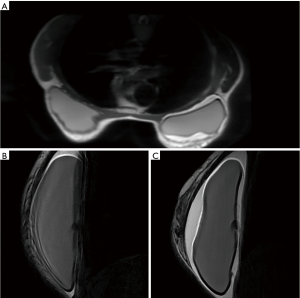
The patient was then referred to a multidisciplinary team with plastic surgeons and hematologists for making a decision on further management and treatment strategy. Following an initial treatment with steroids and antibiotics, the removal of implants and the fibrous capsule around them from both breasts was performed on August, 2017. Histologic examination confirmed the diagnosis of BIA-ALCL in the right breast. Immunohistochemistry (IHC) showed strong CD30 expression in anaplastic cells and negativity of anaplastic lymphoma kinase (ALK). Tumor cells were also positive for CD4, CD43, CD3, CD45, CD2, and negative for CD5, CD7, CD8, and CD15. No lesions were detected in left breast implant.
Staging procedures performed after surgery, including PET/CT and MRI revealed no abnormalities. No additional treatment was proposed, and the patient was found in complete remission 3 months later.
On November, 2017, the patient was referred to our attention for an opinion on the opportunity to proceed with a breast reimplant.
The patient was in excellent physical condition, reported no problems associated with previous surgical treatment. However, she was extremely worried about her aesthetic aspect. The clinical examination confirmed the absence of disease recurrence. Both “empty” breasts had normal aspect, no signs of inflammation, the skin was soft, not fixed on the underlying tissues.
Once we reassured her that the BIA-ALCL was successfully cured with the removal of both implants and, given the limited stage of the disease, the risk of relapse was negligible, she asked our opinion on the risk of a new BIA-ALCL in case of a new breast implant. She confirmed the strong desire in improving the shape of her breasts.
After a detailed analysis of costs, benefits, and taking into account her expectations, we stated that smooth implants could be re-inserted after at least 1 year of follow-up confirming the absence of any recurrence, also informing the patient that the safety of this strategy is still being investigated.
Discussion
In 2016, more than 1,649,271 breast implants worldwide have been reported by the International Society of Aesthetic Plastic Surgery (ISAPS). Among them, breast augmentation with silicone was by far the most adopted procedure with 87.8% of cases. But there are no data about what kind of implants are in use more frequently: textured or smooth. ISAPS survey showed 9% increase of aesthetic procedures compared to 2015 and highlighted top 5 countries with the highest rank of procedures: USA, Brazil, Japan, Italy, Mexico. Breast augmentation is the third fastest growing cosmetic procedure (increased to 22%) and the first between surgical procedures (15.8% of all). From year to year the number of such procedures is increasing due to quality of life improvement (QoL) and psychological aspects. Nevertheless, woman proceeded for breast augmentation for aesthetic or reconstructive reasons could be at risk group for developing BIA-ALCL. According to the worldwide experience in diagnostics and treatment of BIA-ALCL, there were proposed recommendations for all patients who is going to proceed for breast implant surgery. They include implant selection, discussion about all benefits and limitations of implant types, avoiding implant removal from asymptomatic woman, the use of antibacterial strategy (10).
Since FDA published data about 359 cases of BIA-ALCL, there are still no references about possibility and safety of reimplant after implant removal. This strategy requires more detailed investigation, analysis and discussion. According to presented information published last years, there are two main reasons that can be related to each other in the onset of BIA-ALCL: type of implant material and chronic bacterial biofilm infection around implants (8,11).
Out of the 359 cases of BIA-ALCL reported by FDA, there are 231 cases with available information about implant surface. Data showed that 203 cases were with textured implants, while only 28 were smooth, but no significant influence of filled material in implant. This phenomenon should be also explained that only textured implants are available in some countries (5). Other important fact of their worldwide use is a minimal risk of capsular contracture development compared to smooth implants (12).
In addition to above, based on the obtained data, one of the supposed causes of disease may be the presence of specific bacteria biofilm on implant surface. Hu et al. showed specific microbiome of Ralstonia spp. (Gram-negative) in BIA-ALCL specimens compared to Staphylococcus spp. in normal and contracture capsule specimens (8).
Taking into account these results, Adams et al. analyzed the outcome of 42,000 textured implants in 21,650 women who underwent breast augmentation for different reasons (13). Data were prospectively collected from five countries. The main idea was to establish the incidence of BIA-ALCL through the use of the surgical 14-point plan, which was presented and adapted worldwide in 2013 to minimize a level of capsular contractures due to bacterial load during breast augmentation (14,15). Of note, the adoption of the surgical 14-point plan resulted in no cases of BIA-ALCL during follow-up. Despite all data given above, we still need more information about mechanisms of developing BIA-ALCL.
Returning to the issue of possible reimplant in case of these patients, there are still more questions than answers. Some data suggest the safety of reimplant with smooth implants, although the median follow-up is short, and strict monitoring is mandatory (4).
In patients with limited stage BIA-ALCL the risk of relapse after a complete surgical removal is very low. In the report from the MD Anderson Cancer Center, 39 (93%) of 42 patients without a mass achieved complete remission at last follow-up, and 100% of overall survival was reported among 19 cases collected in France from different institutions through Lymphopath over a 5-year period (16).
The Australian and New Zealand BIA-ALCL Task Force has recently provided updated information regarding BIA-ALCL (17). Based on their huge experience and other available information we believe that in patients with limited stage BIA-ALCL asking for reimplant we can say that smooth implants could be re-inserted after at least 1 year of continuous complete remission following surgical removal, also informing the patient that the safety of this strategy is still being investigated.
In conclusion, if we are asked whether reimplant after BIA-ALCL is a safe procedure, based on available data, and upon our own experience we can say “Yes, may be”. Of course, we still are in emergency need for more data coming from existing data bases.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aol.2018.07.01). MF serves as an unpaid editorial board member of Annals of Lymphoma from Jan 2017 to Jan 2019. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Informed consent was obtained from the patient for publication of this case report with any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127:2375-90. [Crossref] [PubMed]
- Keech JA Jr, Creech BJ. Anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plast Reconstr Surg 1997;100:554-5. [Crossref] [PubMed]
- FDA food and drug administration. Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) [Internet]. 2017. Available online: https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/ BreastImplants/ucm239995.htm
- Clemens MW, Nava MB, Rocco N, et al. Understanding rare adverse sequelae of breast implants: anaplastic large-cell lymphoma, late seromas, and double capsules. Gland Surg 2017;6:169-84. [Crossref] [PubMed]
- Leberfinger AN, Behar BJ, Williams NC, et al. Breast Implant-Associated Anaplastic Large Cell Lymphoma: A Systematic Review. JAMA Surg 2017;152:1161-8. [Crossref] [PubMed]
- Australian Government D of H. Breast implants and anaplastic large cell lymphoma [Internet]. 2017. Available online: https://www.tga.gov.au/alert/breast-implants-and-anaplastic-large-cell-lymphoma
- Santanelli di Pompeo F, Laporta R, Sorotos M, et al. Breast Implant-Associated Anaplastic Large Cell Lymphoma: Proposal for a Monitoring Protocol. Plast Reconstr Surg 2015;136:144e-51e. [Crossref] [PubMed]
- Hu H, Johani K, Almatroudi A, et al. Bacterial Biofilm Infection Detected in Breast Implant-Associated Anaplastic Large-Cell Lymphoma. Plast Reconstr Surg 2016;137:1659-69. [Crossref] [PubMed]
- Loch-Wilkinson A, Beath KJ, Knight RJW, et al. Breast Implant-Associated Anaplastic Large Cell Lymphoma in Australia and New Zealand: High-Surface-Area Textured Implants Are Associated with Increased Risk. Plast Reconstr Surg 2017;140:645-54. [Crossref] [PubMed]
- Thompson PA, Prince HM. Breast implant-associated anaplastic large cell lymphoma: a systematic review of the literature and mini-meta analysis. Curr Hematol Malig Rep 2013;8:196-210. [Crossref] [PubMed]
- Adams WP Jr. Discussion: Breast Implant-Associated Anaplastic Large Cell Lymphoma in Australia and New Zealand: High-Surface-Area Textured Implants Are Associated with Increased Risk. Plast Reconstr Surg 2017;140:663-5. [Crossref] [PubMed]
- Headon H, Kasem A, Mokbel K. Capsular Contracture after Breast Augmentation: An Update for Clinical Practice. Arch Plast Surg 2015;42:532-43. [Crossref] [PubMed]
- Adams WP Jr, Culbertson EJ, Deva AK, et al. Macrotextured Breast Implants with Defined Steps to Minimize Bacterial Contamination around the Device: Experience in 42,000 Implants. Plast Reconstr Surg 2017;140:427-31. [Crossref] [PubMed]
- Deva AK, Adams WP Jr, Vickery K. The role of bacterial biofilms in device-associated infection. Plast Reconstr Surg 2013;132:1319-28. [Crossref] [PubMed]
- Clemens MW, Horwitz SM. NCCN Consensus Guidelines for the Diagnosis and Management of Breast Implant-Associated Anaplastic Large Cell Lymphoma. Aesthet Surg J 2017;37:285-9. [Crossref] [PubMed]
- Miranda RN, Aladily TN, Prince HM, et al. Breast implant-associated anaplastic large-cell lymphoma: long-term follow-up of 60 patients. J Clin Oncol 2014;32:114-20. [Crossref] [PubMed]
- Kim B, Roth C, Chung KC, et al. Anaplastic large cell lymphoma and breast implants: a systematic review. Plast Reconstr Surg 2011;127:2141-50. [Crossref] [PubMed]
Cite this article as: Tarantino V, Skypets T, Angrilli F, Federico M. Is reimplant after breast implant-associated anaplastic large cell lymphoma a safe procedure? Yes, no, or may be? Ann Lymphoma 2018;2:6.


