
Aggressive B-cell lymphomas—from morphology to molecular pathogenesis
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma (NHL), representing around 30% to 40% of all newly diagnosed lymphomas. DLBCL is clinically, morphologically and biologically a heterogeneous disease reflected in its highly variable clinical course. The 2016 World Health Organization (WHO) classification of lymphomas (Table 1) recognizes within the group of large B-cell lymphomas several distinct entities characterized by unique clinical and pathological features including primary DLBCL of the central nervous system (CNS), primary cutaneous DLBCL, leg type, primary mediastinal (thymic) large B-cell lymphoma (PMBL), T-cell histiocyte-rich large B-cell lymphoma (TCHRLBCL) and EBV positive DLBCL, NOS (1-8). Nevertheless, most cases of DLBCL fall into the “NOS” category (9). Gene expression profiling (GEP) studies have revealed that DLBCL comprises several molecular groups that reflect either the stage in B cell development from which the disease originates or the activity of different biological programs (10,11). Based on these GEP studies, DLBCLs have been divided into two main groups based on the putative cell of origin (COO). Germinal center B cell-like (GCB)-DLBCL exhibits a transcriptional profile that resembles that of a GCB cell with expression of CD10 and the transcriptional repressor BCL6 and harbouring highly mutated immunoglobulin genes with ongoing somatic hypermutations (SHM). Activated B cell-like (ABC)-DLBCL shows several features of B cell receptor (BCR) activated B-cells with up-regulation of genes required for plasma cell differentiation (IRF4/MUM1). These tumors downregulate the GC-specific program, activating at the same time, the NF-kB and BCR signalling pathways. These activated signalling pathways are crucial to promote cell survival, proliferation, and inhibition of apoptosis (12,13). Consistent with their late GC origin, these tumors do not show evidence of ongoing SHM. A less well-characterized group comprising about 15% of the cases remain unclassifiable. More recently the mutational analysis of DLBCL provided new insights into DLBCL pathogenesis and suggested that these genetic signatures also predict clinical outcome and can be used to develop new treatment strategies (14,15).
Table 1
| DLBCL, NOS |
| Morphological variants |
| Centroblastic |
| Immunoblastic |
| Anaplastic |
| Other rare morphological variants |
| Specific immunophenotype |
| Double-expresser DLBCL, NOS |
| CD30-positive DLBCL, NOS |
| CD5-positive DLBCL, NOS |
| Cyclin D1-positive DLBCL, NOS |
| Molecular subtypes |
| Germinal centre B-cell (GCB) subtype |
| Activated B-cell (ABC) subtype |
| Unclassified by gene expression profiling |
| Primary DLBCL of the CNS |
| Primary cutaneous DLBCL, leg type |
| Primary mediastinal (thymic) large B-cell lymphoma (PMBL) |
| Primary effusion lymphoma (PEL) |
| Intravascular large B-cell lymphoma |
| T-cell/histiocyte-rich large B-cell lymphoma (THRLBCL) |
| Plasmablastic lymphoma (PBL) |
| EBV-positive DLBCL, NOS |
| HHV8-positive DLBCL, NOS* |
| DLBCL associated with chronic inflammation |
| Lymphomatoid granulomatosis |
| ALK-positive large B-cell lymphoma |
| Large B-cell lymphoma with IRF4 rearrangement |
| Burkitt lymphoma |
| Burkitt-like lymphoma with 11q aberration* |
| HGBL with MYC and BCL2 and/or BCL6 rearrangement (i.e., double-hit or triple-hit lymphoma) |
| HGBL, NOS |
| B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and CHL |
*, provisional entity. ALK, anaplastic lymphoma kinase; CHL, classical Hodgkin lymphoma; CNS, central nervous system; DLBCL, diffuse large B-cell lymphoma; EBV, Epstein-Barr virus; HGBL, High-grade B-cell lymphoma; HHV8, human herpesvirus 8; NOS, not otherwise specified.
The distinction between Burkitt lymphoma (BL) and other morphologically aggressive B-cell lymphomas has been problematic for pathologists. GEP studies have shown that BL has a characteristic signature but that there are cases within the spectrum of DLBCL and aggressive B-cell lymphomas, which have a similar BL signature or fall into an intermediate category (16). The 2008 WHO classification recognized this problem and added a provisional category of B cell lymphoma, unclassifiable, with features intermediate between DLBCL and BL (BCLU) (17). The BCLU category was enriched with cases carrying MYC and BCL2 and/or BCL6 translocations so-called double-hit (DHL) and triple-hit (THL) lymphomas (18,19). The 2016 WHO classification substituted the BCLU category with two provisional categories of high-grade B cell lymphomas (HGBL); one with MYC and BCL2 and/or BCL6 rearrangements (DHL and THL), and one HGBL, NOS, characterized by high grade morphology but without translocations (20). These two categories should be recognized because of their worse outcome, potentially different treatment strategy, and different genetic profile compared with BL and DLBCL, NOS (21).
There are still some unsolved or controversial questions about HGBL, such as how to select cases to do FISH analysis for MYC, BCL2 and/or BCL6, the prognostic significance of BCL2-MYC vs. BCL6-MYC DHL and THL, IG and non-IG partner of MYC translocation, and the importance of single-hit lymphoma with MYC translocation only with or without amplification or copy number gains of BCL2 and/or BCL6. In this review, we will focus on DLBCL, NOS and HGBL highlighting some unsolved or controversial issues.
Diffuse large B-cell lymphoma, NOS
DLBCL, NOS, is the most common type of NHL, accounting for 25–35% of adult NHL in developed countries (22). It is a B-cell lymphoma composed of large to medium-sized cells with a diffuse growth pattern, excluding other specific entities listed in Table 1.
Morphology
DLBCL, NOS has three common morphological variants (centroblastic, immunoblastic and anaplastic) and several rare variants (Figure 1). The centroblastic variant is the most common and is characterized by medium-sized to large lymphoid cells with vesicular nuclei containing fine chromatin. There are 2–4 nuclear membrane-bound nucleoli, and scant basophilic cytoplasm. The immunoblastic variant is defined as a tumor with >90% immunoblasts (22). The immunoblasts typically contain a large, oval or round nucleus, a single prominent centrally-located nucleolus, and considerable basophilic cytoplasm. Some cases show overlapping features with plasmablasts with eccentric nuclei and perinuclear hof. These cases should be differentiated from plasmablastic lymphoma, ALK-positive large B-cell lymphoma, Epstein-Barr virus (EBV)-positive DLBCL, NOS, extracavitary primary effusion lymphoma and HHV8-positive DLBCL, NOS. Recently, this morphologic variant was associated with higher IGH/MYC rearrangement frequency without concurrent BCL2 or BCL6 rearrangement (23). The anaplastic morphology is characterized by one to several, large, pleomorphic nuclei, mimicking Hodgkin/Reed-Sternberg cells or the tumor cells of anaplastic large cell lymphoma (ALCL). Some cases show sinusoidal and/or cohesive growth pattern, mimicking ALCL or undifferentiated carcinoma. However, these cases are not associated with ALK translocation, and are not related to ALCL. Other rare morphological variants include sinusoidal CD30-positive DLBCL (24), spindle cell morphology (25), signet ring cell morphology (26), myxoid stroma (27), fibrillary matrix or rosette formation (28), marked tissue eosinophilia (29), and microvillous DLBCL (30). These rare morphological variants are sometimes within the differential diagnoses of ALCL, undifferentiated carcinoma, sarcoma, signet ring cell carcinoma or neurogenic tumors.
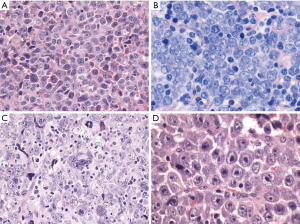
Immunophenotype
Immunophenotypically, the neoplastic cells express pan-B-cell markers, such as CD19, CD20, CD22, CD79a, and PAX5, but may lose one or more of them. The Ki-67 proliferation index is usually high and can be more than 90% in some cases. The immunophenotype and differential diagnosis with other aggressive B-cell lymphomas are summarized in Table 2.
Table 2
| Diagnosis | CD20 | PAX5 | CD10 | BCL6 | MUM1 | BCL2 | MYC | CD138 | CD30 | EBER | HHV8 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| DLBCL, NOS, GCB subtype | + | + | +/− | + | −/+ | +/− | −/+ | − | −/+ | − | − |
| DLBCL, NOS, ABC subtype | + | + | − | +/− | + | +/− | −/+ | − | −/+ | − | − |
| PMBL | + | + | −/+ | +/- | +/− | +/− | −/+ | − | +/− | − | − |
| PBL | − | − | −/+ | − | + | − | +/− | + | −/+ | +/− | − |
| LYG | + | + | NA | NA | NA | NA | NA | NA | +/- | + | − |
| BL | + | + | + | + | −/+ | − | + | − | − | −/+ | − |
| LBCL with IRF4 rearrangement | + | + | +/− | + | + | +/− | NA | NA | NA | − | − |
| ALK+ LBCL | − | − | NA | NA | + | NA | + | + | − | − | − |
| EBV+ DLBCL, NOS | + | + | − | +/− | + | −/+ | +/− | NA | +/- | + | − |
| HHV8+ DLBCL, NOS | +/− | +/− | − | − | + | NA | NA | −/+ | NA | − | + |
| PEL | − | − | − | − | + | − | +/− | +/− | +/− | +/− | + |
+, positive; +/−, mostly positive; −/+, mostly negative; −, negative. ABC, activated B-cell; BL, Burkitt lymphoma; DLBCL, diffuse large B-cell lymphoma; GCB, germinal centre B-cell; LBCL, large B-cell lymphoma; LYG, lymphomatoid granulomatosis; NA, not available; NOS, not otherwise specified; PBL, plasmablastic lymphoma; PEL, primary effusion lymphoma; PMBL, primary mediastinal large B-cell lymphoma.
CD30 is positive in 10–20% of cases of DLBCL, NOS and might be associated with anaplastic morphology (Figure 2). Interestingly, the GEP of CD30+ DLBCL overlaps with that of PMBL, and is associated with a favorable prognosis with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone) therapy, regardless of the COO (31,32). Some recent studies showed that CD30 expression in DLBCL was associated with lack of MYC rearrangement, which might be the real cause of its better prognosis (33,34). Cases with CD30 expression might benefit from anti-CD30 therapy (35), but more large-scaled studies are warranted.
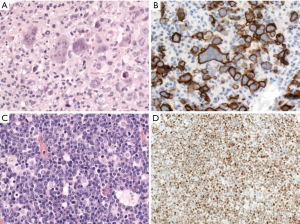
CD5 is positive in about 5–10% of cases of DLBCL, NOS. Most cases are de novo, while the minority of CD5-positive DLBCL cases are transformed from B-chronic lymphocytic leukemia/small lymphocytic lymphoma (so-called Richter transformation). CD5 positivity is associated with higher frequency of bone marrow involvement, CNS relapse, ABC subtype, BCL2 overexpression, STAT3 and NF-kB activation, and worse overall survival in cases of DLBCL, NOS with R-CHOP or R-EPOCH (rituximab, etoposide, prednisone, vincristine, cyclophosphamide and doxorubicin) treatment (36,37).
Cyclin D1 expression has been reported in 1.5–15% of DLBCL cases with about 10% of these cases showing copy number gains of CCND1 gene, but not translocation (Figure 2) (38-46). Cyclin D1-positive DLBCL, NOS, can be distinguished from pleomorphic mantle cell lymphoma (MCL) by lack of CD5 and SOX11 expression and lack of CCND1 translocation (38-46). A similar phenomenon has been reported in plasma cell myeloma (47), and nodular lymphocyte predominant Hodgkin lymphoma (48).
GCB versus non-GCB phenotype
Because the accurate distinction of the GCB from the ABC suptype seems to be an important predictive factor in DLBCL, NOS, the 2016 WHO classification recommends to include this information in the pathological report. GEP, which is considered the gold standard to assign the molecular subtypes, is not routinely available and is not cost-effective. Several studies have attempted to recapitulate the molecular subgroups (GCB vs. non-GCB) using a limited panel of antibodies available in most pathology laboratories. Although most studies find that immunohistochemical algorithms (Hans, Choi or Tally) correlate with prognosis in DLBCL, everybody agrees that these algorithms are an imperfect substitution for GEP. The Hans algorithm has been the most widely used in clinical trials. In this classifier three antibodies are used CD10, BCL6 and IRF4/MUM1 (49). Cases positive for CD10 or cases positive for BCL6 and negative for IRF4/MUM1 are classified as GCB phenotype whereas cases that are IRF4/MUM1 positive with or without expression of BCL6 are assigned to the non-GCB subtype (Figure 3) (49). More recently, mRNA based techniques have emerged as a realistic option to accurately determine the COO (50,51). These techniques have shown to work reliably with formalin-fixed paraffin-embedded tissue.
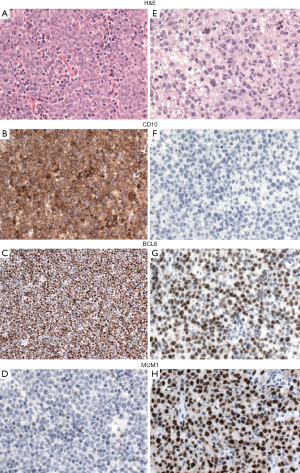
Double-expresser lymphoma
A subset of DLBCL, NOS show co-expression of MYC and BCL2 proteins, without demonstrable MYC or BCL2 translocation. These cases are referred as “double-expresser” lymphomas. BCL2 and MYC protein are positive in about 50% and 30%, respectively, of DLBCL cases with the cut-off value of 50% and 40%, respectively (52). Double-expresser lymphomas account for about 30% of DLBCL, NOS, and they are more commonly of the ABC subtype (2,52,53). Double-expresser lymphomas are associated with worse prognosis when compared with cases that do not express MYC and BCL2 proteins, but better prognosis when compared to DH or TH lymphomas (2,52-54).
COO
According to the 2016 WHO classification, diagnosis of all cases of DLBCL, NOS should include COO, (GCB vs. ABC, or non-GCB if an IHC algorithm is used), because of their different molecular features, biologic behavior, prognosis and treatment (22). ABC subtype shows NF-kB activation and recurrent mutation of MYD88 and CD79B (55), while GCB subtype reveals more frequently BCL2 rearrangement and recurrent mutation of BCL2, TNFRSF14 (56), EZH2 (57), and GNA13 (58). ABC subtype has worse prognosis than GCB subtype with R-CHOP therapy (59). However, cases of ABC subtype seem to benefit from adding lenalidomide (60) or ibrutinib (61) to R-CHOP, reaching a similar prognosis to GCB subtype, but these results need further confirmation. Primary DLBCL of the CNS (62), testis (63), and breast (64), as well as primary cutaneous DLBCL, leg type (65) usually belong to ABC subtype and show more frequently mutations of MYD88 and/or CD79B. New mutational studies have identified a group with frequent alterations in BCL6 (fusion/translocation) and mutations in NOTCH2 but GEP independent from the GCB and ABC subtypes that suggest a derivation from marginal zone cells (14,15).
MYC rearrangement (single-hit lymphoma)
The prognosis and treatment of so-called single-hit lymphoma (SHL) with MYC rearrangement are still inconclusive due to variable morphology (DLBCL or BCLU morphology), presence or absence of gene amplification or copy number gains, and treatment (R-CHOP or more intensive therapy) in previous studies. Some studies showed poor prognosis of SHL similar to DHL (66-69), while others revealed better prognosis than DHL (70). Landsburg et al. found that cases of single-hit DLBCL, NOS with R-CHOP treatment showed poor prognosis similar to DHL, but those with more intensive therapy revealed better prognosis similar to MYC-normal DLBCL, NOS (67). Li et al. reported that SHL had similar poor prognosis to DHL, higher p53 overexpression, less frequent expression of CD10, BCL6 and BCL2, less history of previous low-grade B-cell NHL, and more IGH partner of MYC translocation than DHL (66). The poor prognosis is probably due to p53 mutations, and they suggested SHL be treated as DHL (66).
BL
BL is a highly aggressive but potentially curable mature B-cell lymphoma, characterized by MYC translocation to an IG locus, and simple karyotype. BL is considered to arise from GC B-cells in the dark zone and expresses CD10 and BCL6, but not BCL2. A combination of morphology, immunophenotyping and genetic analysis is necessary for the diagnosis of BL. There are three epidemiological variants of BL. Endemic BL occurs in equatorial Africa and Papua New Guinea and shows strong association with EBV and malaria. It usually presents as a rapidly-growing mass in the jaw and other facial bones of children in endemic areas. Other frequently involved sites include distal ileum, cecum, omentum, gonads, kidneys, long bones, thyroid, salivary glands, and breasts (71). Sporadic BL occurs throughout the world, mainly in children and young adults, but also in the elderly. It usually presents as an abdominal mass, especially in ileocecal region, ovaries, and kidneys. EBV is positive in 20–30% of sporadic BL with variable frequency in different countries (72). Immunodeficiency-associated BL occurs mainly in HIV-infected patients when CD4+ T-cell counts are still high. Nodal and bone marrow involvement is more frequent in immunodeficiency-associated BL than in endemic or sporadic BL. EBV is positive in 25–40% of cases.
Morphology and immunophenotype
BL is characterized by monotonous, medium-sized lymphoma cells with round nuclei, multiple, small, paracentrally located nucleoli, basophilic cytoplasm with squared-off borders, and frequent mitoses with tingible body macrophages and starry sky appearance (Figure 4). Cytoplasmic lipid vacuoles are noted in aspiration or imprint cytology. Greater nuclear pleomorphism and plasmacytoid appearance are noted, especially in HIV-associated cases. The tumor cells typically express pan-B-cell markers (CD19, CD20, CD22, CD79a, and PAX5) and GC B-markers (CD10 and BCL6). Ki-67 proliferation index is almost 100%. MYC is usually expressed. BCL2 should be negative or weakly positive in a minority of cells. High BCL2 expression suggests other lymphomas, especially HGBL with MYC and BCL2 and/or BCL6 rearrangements (DHL or THL). TdT is negative.
Molecular features and FISH analysis
The molecular feature of BL is MYC rearrangement with the partner mostly IGH as t(8;14)(q24;q32) and less commonly IGK as t(2;8)(p12;q24) or IGL as t(8;22)(q24;22q11). However, MYC rearrangement also occurs in other types of lymphoma, including plasmablastic lymphoma, HGBL, and DLBCL, NOS, as well as rare cases of transformed lymphomas (73). In contrast to other lymphomas with MYC rearrangement, BL cases show relatively simple karyotype with no or very few chromosomal aberrations except MYC rearrangement (74). FISH is a sensitive and specific method to detect MYC rearrangement. Around 3–10% of BL cases lack MYC rearrangement detected by FISH or conventional cytogenetics (75,76). A distant breakpoint from MYC gene or small insertion of MYC into IG locus is suggested in some negative cases that may be detected by specifically designed FISH probes (75,77). It is recommended to use several FISH probes both break-apart probes (BAP) and dual-fusion probes (DFP) in cases where BL is suspected and the initial FISH analysis is negative.
Mutational landscape
Mutations in TCF3 and/or its negative regulator ID3 are identified in 70%, 67%, and 40% of sporadic BL, HIV-associated BL, and endemic BL, respectively, but rare in other lymphomas, such as DLBCL (78). Gain-of-function of TCF3 and loss-of-function of ID3 activate B-cell receptor signaling through PI3K pathway, promoting cell survival and proliferation in BL. Besides, oncogenic mutation of CCND3 are found in 38% of sporadic BL cases, producing highly stable cyclin D3 isoforms that drive cell cycle progression (78). In contrast, other recurrent mutations of DLBCL, such as EZH2, SGK1, BCL2, CD79B, and MYD88, are rarely found in BL (78,79).
Burkitt-like lymphoma with 11q aberration
Recently, some cases resembling BL in morphology, immunophenotype, and gene expression profile, but also DLBCL, NOS, lack MYC rearrangement and contain a peculiar chromosome 11q aberration with gain in 11q23.2-23.3 and loss of 11q24.1-qter (76). These cases have more complex karyotypes and more frequent nodal presentation than BL, but the clinical course and prognosis seem to be similar (76). Whether this group of cases should be classified as a molecular variant of BL or a distinct entity is controversial, and it is placed as a provisional entity in the 2016 WHO classification (80). Post-transplant molecularly defined BL cases more frequently have this characteristic 11q-gain/loss pattern and lack of MYC rearrangement than immunocompetent cases (81). The 11q-gain/loss aberration has been found not only in MYC-negative Burkitt-like lymphoma, but also in some cases of MYC-positive BL and MYC-positive HGBL, NOS (82).
HGBL with MYC and BCL2 and/or BCL6 rearrangements
HGBL with MYC and BCL2 and/or BCL6 rearrangements (so-called DHL or THL) is defined as an aggressive mature B-cell lymphoma with coexisting rearrangements of MYC at chromosome 8q24 and BCL2 at chromosome 18q21 and/or BCL6 at chromosome 3q27. Cases with rearrangements of all three loci, MYC, BCL2 and BCL6, are called THL. Some cases of THL show reciprocal translocation of MYC and BCL6 (83). Cases with coexisting rearrangements of BCL2 and BCL6 without MYC rearrangement, and cases with coexisting rearrangements of MYC and other genes such as CCND1 are excluded. MCL with MYC rearrangement (84,85), are per definition excluded from the group of HGBL in the 2016 WHO classification. The translocation partner of MYC can be immunoglobulin (IG) or non-IG gene. Cases with IG/MYC translocation showed worse prognosis than non-IG/MYC translocation in some studies (86,87). Cases with amplification, copy number gains, somatic mutation of genes or increased protein expression, including double-expresser DLBCL, without translocation are excluded although some studies showed poor prognosis in these groups, similar to DH lymphomas (1,54,70,88). Rare cases of B-cell lymphoblastic lymphoma (B-LBL) and pure follicular lymphoma (FL) with MYC and BCL2 rearrangements are also excluded. The classification is primarily applicable to de novo cases; transformed FL with DLBCL component showing MYC and BCL2 rearrangements should be diagnosed as HGBL with MYC and BCL2 rearrangements, transformed from FL. Before the 2016 WHO classification, some studies included cases of B-LBL (1,5), pure FL (1,83,86,89) or MCL (89) with DH as well as translocation in one gene and extra copies in the other gene (1). We should notice that these cases do not fit the current criteria of HGBL with DH/TH in the 2016 WHO classification of lymphomas (21).
Clinical presentation
Most patients present with advanced stage (Ann Arbor III or IV in 84–100% of patients), nodal and extranodal involvement, including bone marrow and CNS, B symptoms, intermediate-high or high International Prognostic Index (IPI) score, and high serum lactate dehydrogenase (LDH) levels (3-6,66,88). Around 30% of cases have a previous history of B cell NHL, most frequently FL, acquiring a secondary MYC rearrangement and transforming to DHL (5,66,88). Rare cases present in malignant effusion without solid mass, similar to primary effusion lymphoma or effusion-based lymphoma (90,91).
Morphology
Around 32% to 69% of the cases showed similar morphology to DLBCL, NOS (5,19,21,86), and about 2–8% of all cases with DLBCL morphology are DH lymphomas (3,4,6,21). The other cases mostly revealed morphologic features of BCLU as defined in the 2008 WHO classification (Figure 5). BCLU cases have morphological features intermediate between DLBCL and BL with diffuse pattern, starry-sky appearance, medium-sized to large tumor cells, slightly irregular nuclear contours, inconspicious or small nucleoli, and scant cytoplasm (Figure 5B). High mitotic activity and apoptosis are frequently found, but still some cases have low number of mitoses. Some cases are relatively monotonous, mimicking BL, but the immunophenotype and genetic findings are different. There are also some cases revealing blastoid morphology similar to B-LBL or blastoid variant of MCL (Figure 5D). Around 60% of HGBL with blastoid morphology showed DH of MYC and BCL2 rearrangements and most of them revealed GCB phenotype with some transformed from FL (92). Besides, blastoid cases are enriched in DHL or THL, and have a significantly worse prognosis even among DHL or THL with other morphologies (70). Cases of MYC-BCL6 DHL and THL are much less common than MYC-BCL2 DHL (4). Around 33–85% of MYC-BCL6 DHL show DLBCL morphology, while 15–67% of cases display BCLU morphology in three larger series (19,91,93). Half of THL reveal DLBCL morphology and the other half show BCLU morphology in one series (83). The comparison of MYC-BCL2 DHL, MYC-BCL6 DHL and THL according to the literature is summarized in Table 3.
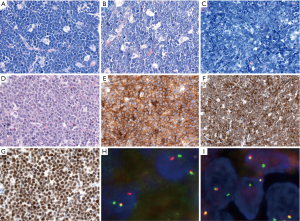
Table 3
| Features | MYC-BCL2 DHL | MYC-BCL6 DHL | THL |
|---|---|---|---|
| Percentage of total DLBCL cases | 2–8% | 0.8–1% | 0.4–3% |
| Percentage of total BCLU cases | 23% | 9% | NA |
| Percentage of total blastoid cases§ | 64%, including all MYC-BCL2 DHL, MYC-BCL6 DHL, and THL cases | ||
| CD10 positivity | 90–100% | 50–75% | 83–100% |
| BCL6 positivity | 82–95% | 86–100% | 70% |
| MUM1 positivity | 18–39% | 17–88% | 50% |
| BCL2 positivity | 90–95% | 17–80% | 100% |
| MYC positivity | 75–84% | 67–100% | 90% |
| DE of MYC/BCL2 | 67–73% | 17–33% | 90% |
| GCB phenotype* | 90-100% | 75–86% | 100% |
| Ki-67 | 20–100% | 40–100% | 75–100% |
| P53 >50% | 33% | NA | NA |
| IG partner of MYC translocation | 56–71% | 31–64% | 53–78% |
| Stage III or IV | 87–100% | 82% | 90% |
| Prognosis compared with DLBCL, NOS | Adverse | Adverse, similar to MYC-BCL2 DHL | Adverse, similar to MYC-BCL2 DHL |
§, blastoid morphology is included in BCLU morphology in some studies; *, GCB or non-GCB phenotype is based on Hans algorithm. BCLU, B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and Burkitt lymphoma; B-NHL, B-cell non-Hodgkin lymphoma; DE, double expression; DHL, double-hit lymphoma; DLBCL, diffuse large B-cell lymphoma; GCB, germinal centre B-cell; NOS, not otherwise specified; THL, triple-hit lymphoma.
Immunophenotype
The lymphoma cells express mature B-cell markers (CD19, CD20, CD79a and PAX5), but are negative for TdT and cyclin D1. MYC-BCL2 DH lymphomas are mostly GCB phenotype (90–100% of cases) (19,91), and almost all these cases express BCL2 (92–95% of cases) (5,19,91), in contrast to BL. Few BCL2-rearranged cases are negative for BCL2 (clone 100D5) due to BCL2 missense mutations, but stain for the BCL2, E17 clone (94). IRF4/MUM1 is positive in 18–39% of MYC-BCL2 DH lymphomas (5,19,91). MYC is positive in 84% of MYC-BCL2 DHLs and 73% of cases show double expression of MYC and BCL2 in one large series (66). The proliferation index is generally high, but highly variable from 20–100% (19), especially in cases with DLBCL morphology. Compared with MYC-BCL2 DHL, cases of MYC-BCL6 DHL express less CD10 (50–75%) and BCL2 (17–80%), but more IRF4/MUM1 (17–88%) (19,91,93). Around 75–86% of MYC-BCL6 DHL cases display GCB phenotype and only 17–33% of cases show double expression of MYC and BCL2, which are less than MYC-BCL2 DHLs (19,91,93). There is a pitfall to misdiagnose MYC-BCL6 DHL cases as BL due to overlapping morphology, common GCB phenotype and BCL2 negativity. The clinical presentation in younger patients, monotonous neoplastic cells without prominent nucleoli, and simple karyotype are clues for BL diagnosis. THL reveal similar immunophenotype than that of DHL with MYC-BCL2 (83). There are some DHL or THL cases that have been reported to be CD20 negative (83,90,91). Epstein-Barr virus (EBV)-encoded small RNA in situ hybridization (EBER) is practically negative with very few exceptions (5,6,90).
How to select cases for FISH analysis?
Currently, there are no perfect criteria to select cases for FISH analysis to detect DHL or THL, especially in cases with DLBCL morphology. Morphologically, cases with blastoid morphology are enriched in DHL or THL (around 60%) (70,92). About 30% and 10% of cases with BCLU morphology are MYC-BCL2 and MYC-BCL6, respectively (18,95). The DHL or THL with DLBCL morphology is the most challenging group (5,19,21,86). Although MYC and BCL2 protein expression correlate with gene translocation in the majority of cases, still around 25% of DHL are negative for MYC using the 40% cut-off value and do not show double expression of MYC and BCL2 (19,54,66,70). The percentage of non-double expression is even lower in MYC-BCL6 DHL (19,93). Therefore, it is believed that a good percentage of DHL are missed by immunohistochemistry. Proliferation index measured by Ki-67 is highly variable (20–100%), and not a good surrogate marker for FISH analysis (19,70). If FISH analysis for MYC and BCL2 is performed in all DLBCL cases with GCB phenotype, most cases of DHL will be identified, but the MYC-BCL6 DH lymphomas will be missed (19,91,93,96). Besides, the specificity is low because around 60% of all DLBCL cases show GCB phenotype (97). Some studies suggest performing FISH analysis in all newly-diagnosed cases of DLBCL (98-100). However, the cost and benefit are difficult to estimate in different institutes or countries. A better strategy might be to start with MYC FISH in cases with aggressive clinical presentation, blastoid or BCLU morphology, double expression of MYC and BCL2, as well as GCB phenotype, and then perform BCL2 and BCL6 if MYC is found to be rearranged.
Break-apart versus DFP
To detect MYC translocation, BAP for MYC gene or DFP of IGH-MYC can be used. BAP shows higher sensitivity than DFP, because of its detection of both IG and non-IG partner, easier interpretation, and probe-design. However, BAP still can result in false negative cases, depending on the probe design and breakpoints (70,77,101). DFP helps identifying the translocation partner of MYC and detect some false-negative cases by BAP (70,77,101). Two-probe approach is optimal, and we suggest at least to start with a BAP due to its higher sensitivity and easier interpretation.
MYC translocation partner: IGH, IG light chain or non-IG gene
The partners of MYC translocation are IG gene in around two-thirds of the cases while non-IG partners are identified in one-third (4,5,66,83,91). The ratio of IGH and IG light chain is highly variable in different studies (5,66,83). Compared with BL, the lower frequency of IGH partner in MYC translocation in DHL or THL suggest that MYC rearrangement is likely a secondary event (66). Although both IG and non-IG partner fulfill diagnosis of DHL or THL in the 2016 revised WHO classification, some studies showed worse prognosis in cases with IG-MYC translocation than non-IG-MYC translocation with R-CHOP treatment (86,87).
Rearrangement versus amplification/copy number gains
According to the 2016 WHO classification, only gene rearrangements fulfill the definition of DHL or THL, but not gene amplification or copy number gains. However, some studies show worse prognosis in DLBCL cases with gene amplification or copy number gains of MYC and BCL2, similar to MYC-BCL2 DHL, especially in cases with coexisting rearrangement in one gene and amplification or copy number gains in the other gene (1,70,88). Li et al. studied cases with coexisting rearrangement or extra copies of MYC and BCL2 and found that these cases had similar poor prognosis to DHL, but more often DLBCL morphology, less frequent CD10 expression and less frequent serum LDH elevation (88). This is still an unresolved issue that warrants further investigation.
Follicular lymphoma with MYC and BCL2 and/or BCL6 rearrangements
Pure FL with MYC and BCL2 and/or BCL6 rearrangements (so-called DH-FL) is excluded from HGBL with MYC and BCL2 and/or BCL6 rearrangements in the 2016 WHO classification. The cases reported in the literature are few with controversial results regarding prognosis and optimal treatment. In two recent series, opposite results were found; one showed poor prognosis of DH-FL, similar to DHL (102), whereas the other study reported an indolent clinical behavior similar to FL without MYC rearrangement, based on clinicopathological and genome-wide copy-number alterations and copy-neutral loss-of-heterozygosity profiles (103). Cases of DH-FL can be low-grade or high-grade, de novo or with high-grade transformation after exclusion of any DLBCL component (104).
Prognosis and treatment
Many studies showed dismal prognosis of HGBL with MYC and BCL2 and/or BCL6 rearrangements under R-CHOP immunochemotherapy, worse than DLBCL, NOS and double-expresser lymphoma (3-6,52,70,100). These patients frequently have several poor prognostic factors, such as elderly patients, advanced stage, bone marrow or CNS involvement, high IPI score or elevated serum LDH level (2-7,70,88). Disease progression or relapse happens frequently. The median overall survival is 1.5 years (3-7). Until now, there is no standard guideline of treatment for these patients. Because R-CHOP immunochemotherapy is thought insufficient for most cases, more intensive therapy, such as R-EPOCH or novel therapy with or without stem cell transplantation should be considered (98,100,101,105,106). CNS prophylaxis is suggested in DHL or THL due to its frequent CNS involvement and relapse (98,100). Some studies showed poor prognosis even with intensive chemotherapy or stem cell transplantation (105,107). Nevertheless, there is a small subset of DHL or THL without risk factors (patients with early stage, low IPI score and low serum LDH level), and these patients seem to have better prognosis (70,106). Some studies showed better prognosis in cases with DLBCL rather than blastoid morphology, without double expression of MYC and BCL2, or with non-IG partner gene of MYC translocation (70,86). Although most studies of DHL were based on MYC-BCL2 DHL, other studies demonstrated poor prognosis of MYC-BCL6 DHL and THL similar to MYC-BCL2 DHL, and these cases should be treated as MYC-BCL2 DHL (19,70,83).
HGBL, NOS
HGBL, NOS is defined as aggressive B-cell lymphoma that lacks MYC, BCL2 and BCL6 rearrangements and morphologically does not fall into the categories of DLBCL, NOS or BL (21). This new provisional category includes cases of BCLU or blastoid morphology without DH or TH. DLBCL, NOS with single hit of MYC or BL with slightly atypical morphology or immunophenotype are excluded. Burkitt-like lymphoma with 11q aberration is also excluded from HGBL, NOS.
Clinical features
There are limited data of HGBL, NOS because most studies lumped cases of HGBL, NOS with DH, TH, or DLBCL, NOS. HGBL, NOS seems to be a heterogeneous group, and the majority of cases have older age, advanced stage, high IPI score and elevated serum LDH level (18,108,109).
Morphology and immunophenotype
Morphologically, HGBL, NOS, should show high grade morphology including BCLU and blastoid morphology. Cases with blastoid morphology might look like BL, but have atypical immunophenotype (BCL2 positivity) or complex karyotype, which do not fit for BL. Cases with DLBCL morphology and high proliferation rates or with MYC as single alteration should be still diagnosed as DLBCL, NOS. The lymphoma cells express mature B-cell markers, but are negative for TdT and cyclin D1 to exclude B-LBL and MCL, respectively, especially in cases with blastoid morphology. HGBL, NOS is a heterogeneous group and mostly shows GCB phenotype with expression of CD10 and BCL6, but less IRF4/MUM1 (18,92,108). Proliferation index of Ki-67 is usually high, but not 100% as in BL (92,108). BCL2 and MYC expression are variable (108).
Molecular features and FISH analysis
To make a definite diagnosis of HGBL, NOS, one should perform FISH of MYC with or without BCL2 and BCL6 to exclude HGBL with MYC and BCL2 and/or BCL6 rearrangements. The genetic findings of HGBL, NOS, are not well studied and seem to be variable, including only MYC, only BCL2 or only BCL6 rearrangement, with or without extra copies, or no abnormalities (18,109). Recently, few cases of HGBL, NOS with MYC rearrangement and 11q aberration were discovered (82,110). Although these cases are different from Burkitt-like lymphoma with 11q aberration because they carry MYC rearrangement, further studies are warranted to see whether these are different diseases or represent a spectrum within HGBL.
Prognosis and treatment
The prognosis of HGBL, NOS is worse than DLBCL or BL (109). Compared with DHL, some studies showed better prognosis in HGBL, NOS (108), while others revealed similar dismal outcome (18), especially in cases with MYC rearrangement (SHL) (66). Patients with age more than 60 years, stage IV or high IPI score have worse prognosis (18,108). Currently, there is no standard treatment for cases of HGBL, NOS. Because of the poor outcome in patients with R-CHOP therapy, alternative treatment should be considered, especially in cases with MYC rearrangement (66,95).
Primary DLBCL of the CNS
Primary DLBCL of the CNS is defined as DLBCL arising in the brain, spinal cord, leptomeninges or eye. It shows similar morphology to DLBCL, NOS, but more frequent perivascular growth pattern and geographic necrosis. Marked tumor necrosis and histiocytic infiltration are often seen after steroid use, causing diagnostic difficulties. Immunohistochemically, it usually shows a non-GCB phenotype. Double expression of BCL2 and MYC is seen in about 80% of cases, but translocations of MYC or BCL2 are rare (111). Primary DLBCL of the CNS shows more frequent recurrent mutation of MYD88 and/or CD79B than nodal DLBCL, NOS (62).
Primary mediastinal (thymic) large B-cell lymphoma (PMBL)
PMBL is a specific type of DLBCL of putative thymic B-cell origin arising in the mediastinum (112). Morphologically, it comprises medium-sized to large centroblastic cells with moderate amount of pale or clear cytoplasm. Although the tumor grows diffusely, collagenous fibrosis compartmentalizing the tumor cells is frequently observed. Immunohistochemically, the tumor cells often express pan-B-cell markers but lack the expression of immunoglobulins despite a functional IG gene rearrangement and the expression of the transcription factors PAX5, OCT2, BOB1 and PU1. The characteristic immunophenotype includes expression of CD23, CD30 and MAL, with variable expression of BCL2, BCL6 and CD10. Rearrangements and mutations in the class II major histocompatibility complex (MHC) transactivator CIITA at 16p13 have been reported in half of the cases resulting in downregulation of MHC class II (113). The unique overexpression of PD-L1 and PD-L2 in PMBL results from the translocation of PDL1 and PDL2 with CIITA or by gene amplification of chromosome 9p24.1 including the JAK2/PDL1/PDL2 locus (114). PMBL is characterized by a constitutively activated NF-kB pathway due, in part, to mutations in TNFAIP3 gene found in up to 60% of cases (56). In addition, these tumors have a constitutively activated JAK/STAT signaling pathway frequently related to inactivating mutations in SOCS1, STAT6 and PTPN1 genes, which are rare or almost absent in DLBCL (115). XPO1 mutations have been described also to be characteristic of PMBL, unlike DLBCL (116). PMBL has a distinct gene expression profile (GEP), which is different from DLBCL, not otherwise specified (NOS), but similar to classic Hodgkin lymphoma (CHL) (117). Interestingly, primary nodal cases without mediastinal involvement with the typical morphology, phenotype and GEP of PMBL have been recently described (112), indicating that rare cases outside the mediastinum do exist. Cases with aberrant cyclin D1 expression due to copy number gains of CCND1 gene have recently been described (118).
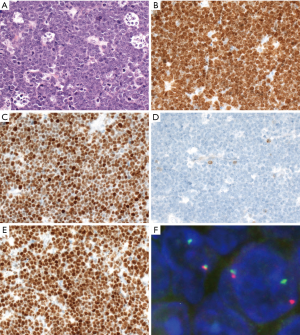
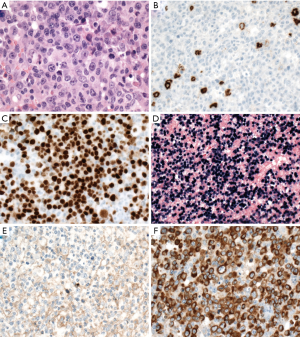
Plasmablastic lymphoma (PBL)
PBL is characterized by plasmablastic or immunoblastic morphology and plasmacytic immunophenotype with expression of CD38, CD138, IRF4/MUM1, BLIMP1/PRDM1, and XBP1, but lack of CD20 and PAX5 (Figure 6). CD79a is positive in about 40% of cases (119). Cytoplasmic immunoglobulin is commonly expressed with either kappa or lambda restriction. Of note, CD10 can be positive in 20% of cases (119), and aberrant T-cell markers such as CD3 might be positive, misleading to a diagnosis of T-cell lymphoma (120). EBER is positive in 65% of cases, especially in HIV-positive patients (119). HHV8 and ALK are negative. MYC translocation is present in about 50% of cases with IG gene as translocation partner, in most cases, and MYC protein overexpression (119,121,122). The morphology and immunophenotype of PBL might overlap with plasmablastic plasma cell myeloma (123). Other differential diagnoses include DLBCL, NOS with loss of CD20 expression, ALK-positive DLBCL, extracavitary PEL, and HHV8-positive DLBCL, NOS.
EBV-positive DLBCL, NOS
EBV-positive DLBCL, NOS, is the current nomenclature in the 2016 WHO, to stress that these lymphomas affect not only elderly patients but also younger patients (124). It is defined as a DLBCL with EBV positivity in >80% of tumor cells. Excluded from this category are other well-characterized EBV-associated entities, such as lymphomatoid granulomatosis, PEL, PBL, DLBCL associated with chronic inflammation, EBV-positive mucocutaneous ulcer, and post-transplant or immunodeficiency-associated EBV-positive lymphoproliferative disorders. EBV-positive DLBCL accounts for 8–15% of DLBCL among Asian and Latin American patients with only 2–3% among Western patients (125). Immunohistochemically, the neoplastic cells usually express pan-B-cell markers, as well as CD30 (124,126). EBV-positive DLBCL, NOS typically has a non-GCB phenotype and a morphology mimicking CHL or THRLBCL (124,126). EBER is positive in all cases with latency II and rarely latency III EBV pattern (124,126). Expression of PDL1 protein (124) and copy number gains of chromosome 9p24.1, containing PDL2 gene (127), are noted, implying immune escape mechanism in its pathogenesis.
Conclusions
The understanding of the biology of DLBCL, BL and HGBL has increased in the last years. The diagnosis of DLBCL needs, in addition to standard morphology and immunohistochemistry, all available ancillary techniques. According to the 2016 WHO classification, the diagnosis of DLBCL, NOS requires the inclusion of the COO (GCB or ABC/non-GCB subtype) determined either with molecular techniques (GEP and mRNA based techniques) or immunohistochemistry, as an alternative solution. The distinction of GCB versus ABC-DLBCL has not yet led to differences in primary treatment. The current standard of care for most patients is R-CHOP, which has improved dramatically the outcome of DLBCL. However, for patients who fail R-CHOP, the choice of therapy is very likely to be influenced by the COO and the molecular pathways used by the tumors for survival and proliferation. Emerging new targeted therapy will certainly influence the diagnosis and treatment of DLBCL and HGBL in the near future. The routine use of FISH and IHC to detect MYC and BCL2 alterations/overexpression is recommended. Patients with DHL and double expression of MYC and BCL2 protein, represent poor-risk subsets in which alternative strategies should be explored. HGBL with MYC and BCL2 and/or BCL6 rearrangements (i.e., DHL or THL) should be separated from DLBCL, NOS, due to its clinicopathological features, molecular findings, and dismal prognosis with standard R-CHOP therapy. Although there are no strict recommendations in how to select cases for FISH analysis, a reasonable approach is to perform FISH analysis for MYC, BCL2 and/or BCL6 in cases with aggressive clinical presentation, blastoid or BCLU morphology, GCB phenotype, and double expression of MYC and BCL2.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Astrid Pavlovsky) for the series “Diffuse Large B-Cell Lymphoma” published in Annals of Lymphoma. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aol.2018.12.02). The series “Diffuse Large B-Cell Lymphoma” was commissioned by the editorial office without any funding or sponsorship. Elias Campo reports grants from NIH, grants from Spanish Ministry of Science, grants from Generalitat de Catalunya, grants from Spanish Ministry of Health, grants and personal fees from Generalitat de Catalunya, during the conduct of the study, personal fees from Takeda, personal fees from Ilumina, personal fees from NanoString, personal fees from Janssen, personal fees from Roche, grants and personal fees from Gilead, grants from European Research Council, outside the submitted work. In addition, Elias Campo has a patent NanoString Technologies licensed. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Li S, Lin P, Fayad LE, et al. B-cell lymphomas with MYC/8q24 rearrangements and IGH@BCL2/t(14;18)(q32;q21): an aggressive disease with heterogeneous histology, germinal center B-cell immunophenotype and poor outcome. Mod Pathol 2012;25:145-56. [Crossref] [PubMed]
- Green TM, Young KH, Visco C, et al. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol 2012;30:3460-7. [Crossref] [PubMed]
- Akyurek N, Uner A, Benekli M, et al. Prognostic significance of MYC, BCL2, and BCL6 rearrangements in patients with diffuse large B-cell lymphoma treated with cyclophosphamide, doxorubicin, vincristine, and prednisone plus rituximab. Cancer 2012;118:4173-83. [Crossref] [PubMed]
- Aukema SM, Siebert R, Schuuring E, et al. Double-hit B-cell lymphomas. Blood 2011;117:2319-31. [Crossref] [PubMed]
- Snuderl M, Kolman OK, Chen YB, et al. B-cell lymphomas with concurrent IGH-BCL2 and MYC rearrangements are aggressive neoplasms with clinical and pathologic features distinct from Burkitt lymphoma and diffuse large B-cell lymphoma. Am J Surg Pathol 2010;34:327-40. [Crossref] [PubMed]
- Niitsu N, Okamoto M, Miura I, et al. Clinical features and prognosis of de novo diffuse large B-cell lymphoma with t(14;18) and 8q24/c-MYC translocations. Leukemia 2009;23:777-83. [Crossref] [PubMed]
- Tomita N, Tokunaka M, Nakamura N, et al. Clinicopathological features of lymphoma/leukemia patients carrying both BCL2 and MYC translocations. Haematologica 2009;94:935-43. [Crossref] [PubMed]
- Kanungo A, Medeiros LJ, Abruzzo LV, et al. Lymphoid neoplasms associated with concurrent t(14;18) and 8q24/c-MYC translocation generally have a poor prognosis. Mod Pathol 2006;19:25-33. [Crossref] [PubMed]
- Pasqualucci L. The genetic basis of diffuse large B-cell lymphoma. Curr Opin Hematol 2013;20:336-44. [Crossref] [PubMed]
- Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000;403:503-11. [Crossref] [PubMed]
- Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med 2002;346:1937-47. [Crossref] [PubMed]
- Davis RE, Ngo VN, Lenz G, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature 2010;463:88-92. [Crossref] [PubMed]
- Lenz G, Staudt LM. Aggressive lymphomas. N Engl J Med 2010;362:1417-29. [Crossref] [PubMed]
- Chapuy B, Stewart C, Dunford AJ, et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med 2018;24:679-90. [Crossref] [PubMed]
- Schmitz R, Wright GW, Huang DW, et al. Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N Engl J Med 2018;378:1396-407. [Crossref] [PubMed]
- Hummel M, Bentink S, Berger H, et al. A biologic definition of Burkitt's lymphoma from transcriptional and genomic profiling. N Engl J Med 2006;354:2419-30. [Crossref] [PubMed]
- Swerdlow SH, Webber SA, Chadburn A, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues: Post-transplant lymphoproliferative disorders. Fourth ed. Lyon: International Agency for Research on Cancer (IARC), 2008.
- Perry AM, Crockett D, Dave BJ, et al. B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and Burkitt lymphoma: study of 39 cases. Br J Haematol 2013;162:40-9. [Crossref] [PubMed]
- Li S, Desai P, Lin P, et al. MYC/BCL6 double-hit lymphoma (DHL): a tumour associated with an aggressive clinical course and poor prognosis. Histopathology 2016;68:1090-8. [Crossref] [PubMed]
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127:2375-90. [Crossref] [PubMed]
- Kluin PM, Harris NL, Stein H, et al. High-grade B-cell lymphoma In: Swerdlow SH, Campo E, Harris NL, et al. editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Revised fourth ed. Lyon: International Agency for Research on Cancer (IARC), 2017;335-41.
- Gascoyne RD, Campo E, Jaffe ES, et al. Diffuse large B-cell lymphoma, NOS In: Swerdlow SH, Campo E, Harris NL, et al. editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Revised fourth ed. Lyon: International Agency for Research on Cancer (IARC), 2017:291-7.
- Horn H, Staiger AM, Vohringer M, et al. Diffuse large B-cell lymphomas of immunoblastic type are a major reservoir for MYC-IGH translocations. Am J Surg Pathol 2015;39:61-6. [Crossref] [PubMed]
- Lai R, Medeiros LJ, Dabbagh L, et al. Sinusoidal CD30-positive large B-cell lymphoma: a morphologic mimic of anaplastic large cell lymphoma. Mod Pathol 2000;13:223-8. [Crossref] [PubMed]
- Carbone A, Gloghini A, Libra M, et al. A spindle cell variant of diffuse large B-cell lymphoma possesses genotypic and phenotypic markers characteristic of a germinal center B-cell origin. Mod Pathol 2006;19:299-306. [Crossref] [PubMed]
- Zhang S, Sun J, Fang Y, et al. Signet-ring cell lymphoma: clinicopathologic, immunohistochemical, and fluorescence in situ hybridization studies of 7 cases. Ann Diagn Pathol 2017;26:38-42. [Crossref] [PubMed]
- Tse CC, Chan JK, Yuen RW, et al. Malignant lymphoma with myxoid stroma: a new pattern in need of recognition. Histopathology 1991;18:31-5. [Crossref] [PubMed]
- Goteri G, Costagliola A, Tassetti A, et al. Diffuse large B-cell lymphoma with Homer-Wright rosettes, sinusoidal growth pattern, and CD30 expression: a possible overlap between microvillous lymphomas and sinusoidal CD30-positive large B-cell lymphomas. Pathol Res Pract 2009;205:279-82. [Crossref] [PubMed]
- Navarro-Román L, Medeiros LJ, Kingma DW, et al. Malignant lymphomas of B-cell lineage with marked tissue eosinophilia. A report of five cases. Am J Surg Pathol 1994;18:347-56. [Crossref] [PubMed]
- Liu A, Sugisaki Y, Hosone M, et al. CD30-positive diffuse large B-cell lymphoma with microvillous features: so-called microvillous lymphoma. J Clin Pathol 2009;62:840-4. [Crossref] [PubMed]
- Hu S, Xu-Monette ZY, Balasubramanyam A, et al. CD30 expression defines a novel subgroup of diffuse large B-cell lymphoma with favorable prognosis and distinct gene expression signature: a report from the International DLBCL Rituximab-CHOP Consortium Program Study. Blood 2013;121:2715-24. [Crossref] [PubMed]
- Slack GW, Steidl C, Sehn LH, et al. CD30 expression in de novo diffuse large B-cell lymphoma: a population-based study from British Columbia. Br J Haematol 2014;167:608-17. [Crossref] [PubMed]
- Wang XJ, Seegmiller AC, Reddy NM, et al. CD30 expression and its correlation with MYC rearrangement in de novo diffuse large B-cell lymphoma. Eur J Haematol 2016;97:39-47. [Crossref] [PubMed]
- Xu J, Oki Y, Saksena A, et al. CD30 expression and prognostic significance in R-EPOCH-treated patients with diffuse large B-cell lymphoma. Hum Pathol 2017;60:160-6. [Crossref] [PubMed]
- Jacobsen ED, Sharman JP, Oki Y, et al. Brentuximab vedotin demonstrates objective responses in a phase 2 study of relapsed/refractory DLBCL with variable CD30 expression. Blood 2015;125:1394-402. [Crossref] [PubMed]
- Xu-Monette ZY, Tu M, Jabbar KJ, et al. Clinical and biological significance of de novo CD5+ diffuse large B-cell lymphoma in Western countries. Oncotarget 2015;6:5615-33. [Crossref] [PubMed]
- Thakral B, Medeiros LJ, Desai P, et al. Prognostic impact of CD5 expression in diffuse large B-cell lymphoma in patients treated with rituximab-EPOCH. Eur J Haematol 2017;98:415-21. [Crossref] [PubMed]
- Ehinger M, Linderoth J, Christensson B, et al. A subset of CD5- diffuse large B-cell lymphomas expresses nuclear cyclin D1 with aberrations at the CCND1 locus. Am J Clin Pathol 2008;129:630-8. [Crossref] [PubMed]
- Rodriguez-Justo M, Huang Y, Ye H, et al. Cyclin D1-positive diffuse large B-cell lymphoma. Histopathology 2008;52:900-3. [Crossref] [PubMed]
- Izquierdo F, Suarez D. CD5(-) diffuse large B-cell lymphoma with peculiar cyclin D1+ phenotype. Pathologic and molecular characterization of a single case. Hum Pathol 2012;43:1344-5. [Crossref] [PubMed]
- Teruya-Feldstein J, Gopalan A, Moskowitz CH. CD5 negative, Cyclin D1-positive diffuse large B-cell lymphoma (DLBCL) presenting as ruptured spleen. Appl Immunohistochem Mol Morphol 2009;17:255-8. [Crossref] [PubMed]
- Schneider A, Meyer P, DiMaio D, et al. Diffuse large B-cell lymphoma with both CD5 and cyclin D1 expression—a case report and review of the literature. Journal of Hematopathology 2010;3:145-8. [Crossref]
- Metcalf RA, Zhao S, Anderson MW, et al. Characterization of D-cyclin proteins in hematolymphoid neoplasms: lack of specificity of cyclin-D2 and D3 expression in lymphoma subtypes. Mod Pathol 2010;23:420-33. [Crossref] [PubMed]
- Vela-Chávez T, Adam P, Kremer M, et al. Cyclin D1 positive diffuse large B-cell lymphoma is a post-germinal center-type lymphoma without alterations in the CCND1 gene locus. Leuk Lymphoma 2011;52:458-66. [Crossref] [PubMed]
- Lucioni M, Novara F, Riboni R, et al. CD5(-) diffuse large B-cell lymphoma with peculiar cyclin D1+ phenotype. Pathologic and molecular characterization of a single case. Hum Pathol 2011;42:1204-8. [Crossref] [PubMed]
- Hsiao SC, Cortada IR, Colomo L, et al. SOX11 is useful in differentiating cyclin D1-positive diffuse large B-cell lymphoma from mantle cell lymphoma. Histopathology 2012;61:685-93. [Crossref] [PubMed]
- Specht K, Haralambieva E, Bink K, et al. Different mechanisms of cyclin D1 overexpression in multiple myeloma revealed by fluorescence in situ hybridization and quantitative analysis of mRNA levels. Blood 2004;104:1120-6. [Crossref] [PubMed]
- Cho BB, Kelting SM, Gru AA, et al. Cyclin D1 expression and polysomy in lymphocyte-predominant cells of nodular lymphocyte-predominant Hodgkin lymphoma. Ann Diagn Pathol 2017;26:10-5. [Crossref] [PubMed]
- Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004;103:275-82. [Crossref] [PubMed]
- Scott DW, Wright GW, Williams PM, et al. Determining cell-of-origin subtypes of diffuse large B-cell lymphoma using gene expression in formalin-fixed paraffin-embedded tissue. Blood 2014;123:1214-7. [Crossref] [PubMed]
- Bobée V, Ruminy P, Marchand V, et al. Determination of Molecular Subtypes of Diffuse Large B-Cell Lymphoma Using a Reverse Transcriptase Multiplex Ligation-Dependent Probe Amplification Classifier: A CALYM Study. J Mol Diagn 2017;19:892-904. [Crossref] [PubMed]
- Johnson NA, Slack GW, Savage KJ, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol 2012;30:3452-9. [Crossref] [PubMed]
- Hu S, Xu-Monette ZY, Tzankov A, et al. MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program. Blood 2013;121:4021-4031; quiz 4250. [Crossref] [PubMed]
- Wang XJ, Medeiros LJ, Lin P, et al. MYC cytogenetic status correlates with expression and has prognostic significance in patients with MYC/BCL2 protein double-positive diffuse large B-cell lymphoma. Am J Surg Pathol 2015;39:1250-8. [Crossref] [PubMed]
- Knittel G, Liedgens P, Korovkina D, et al. Rewired NFkappaB signaling as a potentially actionable feature of activated B-cell-like diffuse large B-cell lymphoma. Eur J Haematol 2016;97:499-510. [Crossref] [PubMed]
- Dubois S, Viailly PJ, Mareschal S, et al. Next-Generation Sequencing in Diffuse Large B-Cell Lymphoma Highlights Molecular Divergence and Therapeutic Opportunities: a LYSA Study. Clin Cancer Res 2016;22:2919-28. [Crossref] [PubMed]
- Morin RD, Johnson NA, Severson TM, et al. Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat Genet 2010;42:181-5. [Crossref] [PubMed]
- Morin RD, Mungall K, Pleasance E, et al. Mutational and structural analysis of diffuse large B-cell lymphoma using whole-genome sequencing. Blood 2013;122:1256-65. [Crossref] [PubMed]
- Fu K, Weisenburger DD, Choi WW, et al. Addition of rituximab to standard chemotherapy improves the survival of both the germinal center B-cell-like and non-germinal center B-cell-like subtypes of diffuse large B-cell lymphoma. J Clin Oncol 2008;26:4587-94. [Crossref] [PubMed]
- Nowakowski GS, LaPlant B, Macon WR, et al. Lenalidomide combined with R-CHOP overcomes negative prognostic impact of non-germinal center B-cell phenotype in newly diagnosed diffuse large B-Cell lymphoma: a phase II study. J Clin Oncol 2015;33:251-7. [Crossref] [PubMed]
- Younes A, Thieblemont C, Morschhauser F, et al. Combination of ibrutinib with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) for treatment-naive patients with CD20-positive B-cell non-Hodgkin lymphoma: a non-randomised, phase 1b study. Lancet Oncol 2014;15:1019-26. [Crossref] [PubMed]
- Zheng M, Perry AM, Bierman P, et al. Frequency of MYD88 and CD79B mutations, and MGMT methylation in primary central nervous system diffuse large B-cell lymphoma. Neuropathology 2017;37:509-16. [Crossref] [PubMed]
- Oishi N, Kondo T, Nakazawa T, et al. High prevalence of the MYD88 mutation in testicular lymphoma: Immunohistochemical and genetic analyses. Pathol Int 2015;65:528-35. [Crossref] [PubMed]
- Taniguchi K, Takata K, Chuang SS, et al. Frequent MYD88 L265P and CD79B Mutations in Primary Breast Diffuse Large B-Cell Lymphoma. Am J Surg Pathol 2016;40:324-34. [Crossref] [PubMed]
- Mareschal S, Pham-Ledard A, Viailly PJ, et al. Identification of Somatic Mutations in Primary Cutaneous Diffuse Large B-Cell Lymphoma, Leg Type by Massive Parallel Sequencing. J Invest Dermatol 2017;137:1984-94. [Crossref] [PubMed]
- Li S, Weiss VL, Wang XJ, et al. High-grade B-cell Lymphoma With MYC Rearrangement and Without BCL2 and BCL6 Rearrangements Is Associated With High P53 Expression and a Poor Prognosis. Am J Surg Pathol 2016;40:253-61. [PubMed]
- Landsburg DJ, Falkiewicz MK, Petrich AM, et al. Sole rearrangement but not amplification of MYC is associated with a poor prognosis in patients with diffuse large B cell lymphoma and B cell lymphoma unclassifiable. Br J Haematol 2016;175:631-40. [Crossref] [PubMed]
- Aukema SM, Kreuz M, Kohler CW, et al. Biological characterization of adult MYC-translocation-positive mature B-cell lymphomas other than molecular Burkitt lymphoma. Haematologica 2014;99:726-35. [Crossref] [PubMed]
- Valera A, Lopez-Guillermo A, Cardesa-Salzmann T, et al. MYC protein expression and genetic alterations have prognostic impact in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Haematologica 2013;98:1554-62. [Crossref] [PubMed]
- Moore EM, Aggarwal N, Surti U, et al. Further Exploration of the Complexities of Large B-Cell Lymphomas With MYC Abnormalities and the Importance of a Blastoid Morphology. Am J Surg Pathol 2017;41:1155-66. [Crossref] [PubMed]
- Leoncini L, Campo E, Stein H, et al. Burkitt lymphoma In: Swerdlow SH, Campo E, Harris NL, et al. editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Revised fourth ed. Lyon: International Agency for Research on Cancer (IARC), 2017;330-334.
- Chen BJ, Chang ST, Weng SF, et al. EBV-associated Burkitt lymphoma in Taiwan is not age-related. Leuk Lymphoma 2016;57:644-53. [Crossref] [PubMed]
- Ott G, Rosenwald A, Campo E. Understanding MYC-driven aggressive B-cell lymphomas: pathogenesis and classification. Hematology Am Soc Hematol Educ Program 2013;2013:575-83. [Crossref] [PubMed]
- Seegmiller AC, Garcia R, Huang R, et al. Simple karyotype and bcl-6 expression predict a diagnosis of Burkitt lymphoma and better survival in IG-MYC rearranged high-grade B-cell lymphomas. Mod Pathol 2010;23:909-20. [Crossref] [PubMed]
- Haralambieva E, Schuuring E, Rosati S, et al. Interphase fluorescence in situ hybridization for detection of 8q24/MYC breakpoints on routine histologic sections: validation in Burkitt lymphomas from three geographic regions. Genes Chromosomes Cancer 2004;40:10-8. [Crossref] [PubMed]
- Salaverria I, Martin-Guerrero I, Wagener R, et al. A recurrent 11q aberration pattern characterizes a subset of MYC-negative high-grade B-cell lymphomas resembling Burkitt lymphoma. Blood 2014;123:1187-98. [Crossref] [PubMed]
- Muñoz-Mármol AM, Sanz C, Tapia G, et al. MYC status determination in aggressive B-cell lymphoma: the impact of FISH probe selection. Histopathology 2013;63:418-24. [Crossref] [PubMed]
- Schmitz R, Young RM, Ceribelli M, et al. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature 2012;490:116-20. [Crossref] [PubMed]
- Love C, Sun Z, Jima D, et al. The genetic landscape of mutations in Burkitt lymphoma. Nat Genet 2012;44:1321-5. [Crossref] [PubMed]
- Leoncini L, Campo E, Stein H, et al. Burkitt-like lymphoma with 11q aberration In: Swerdlow SH, Campo E, Harris NL, et al. editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Revised fourth ed. Lyon: International Agency for Research on Cancer (IARC), 2017;334.
- Ferreiro JF, Morscio J, Dierickx D, et al. Post-transplant molecularly defined Burkitt lymphomas are frequently MYC-negative and characterized by the 11q-gain/loss pattern. Haematologica 2015;100:e275-e279. [Crossref] [PubMed]
- Grygalewicz B, Woroniecka R, Rymkiewicz G, et al. The 11q-Gain/Loss Aberration Occurs Recurrently in MYC-Negative Burkitt-like Lymphoma With 11q Aberration, as Well as MYC-Positive Burkitt Lymphoma and MYC-Positive High-Grade B-Cell Lymphoma, NOS. Am J Clin Pathol 2017;149:17-28. [Crossref] [PubMed]
- Wang W, Hu S, Lu X, et al. Triple-hit B-cell Lymphoma With MYC, BCL2, and BCL6 Translocations/Rearrangements: Clinicopathologic Features of 11 Cases. Am J Surg Pathol 2015;39:1132-9. [Crossref] [PubMed]
- Hu Z, Medeiros LJ, Chen Z, et al. Mantle Cell Lymphoma With MYC Rearrangement: A Report of 17 Patients. Am J Surg Pathol 2017;41:216-24. [Crossref] [PubMed]
- Setoodeh R, Schwartz S, Papenhausen P, et al. Double-hit mantle cell lymphoma with MYC gene rearrangement or amplification: a report of four cases and review of the literature. Int J Clin Exp Pathol 2013;6:155-67. [PubMed]
- Johnson NA, Savage KJ, Ludkovski O, et al. Lymphomas with concurrent BCL2 and MYC translocations: the critical factors associated with survival. Blood 2009;114:2273-9. [Crossref] [PubMed]
- Pedersen MØ, Gang AO, Poulsen TS, et al. MYC translocation partner gene determines survival of patients with large B-cell lymphoma with MYC- or double-hit MYC/BCL2 translocations. Eur J Haematol 2014;92:42-8. [Crossref] [PubMed]
- Li S, Seegmiller AC, Lin P, et al. B-cell lymphomas with concurrent MYC and BCL2 abnormalities other than translocations behave similarly to MYC/BCL2 double-hit lymphomas. Mod Pathol 2015;28:208-17. [Crossref] [PubMed]
- Yoshida M, Ichikawa A, Miyoshi H, et al. Clinicopathological features of double-hit B-cell lymphomas with MYC and BCL2, BCL6 or CCND1 rearrangements. Pathol Int 2015;65:519-27. [Crossref] [PubMed]
- Chen BJ, Chen DY, Kuo CC, et al. EBV-associated but HHV8-unrelated double-hit effusion-based lymphoma. Diagn Cytopathol 2017;45:257-61. [Crossref] [PubMed]
- Pillai RK, Sathanoori M, Van Oss SB, et al. Double-hit B-cell lymphomas with BCL6 and MYC translocations are aggressive, frequently extranodal lymphomas distinct from BCL2 double-hit B-cell lymphomas. Am J Surg Pathol 2013;37:323-32. [Crossref] [PubMed]
- Kanagal-Shamanna R, Medeiros LJ, Lu G, et al. High-grade B cell lymphoma, unclassifiable, with blastoid features: an unusual morphological subgroup associated frequently with BCL2 and/or MYC gene rearrangements and a poor prognosis. Histopathology 2012;61:945-54. [Crossref] [PubMed]
- Turakhia SK, Hill BT, Dufresne SD, et al. Aggressive B-cell lymphomas with translocations involving BCL6 and MYC have distinct clinical-pathologic characteristics. Am J Clin Pathol 2014;142:339-46. [Crossref] [PubMed]
- Adam P, Baumann R, Schmidt J, et al. The BCL2 E17 and SP66 antibodies discriminate 2 immunophenotypically and genetically distinct subgroups of conventionally BCL2-"negative" grade 1/2 follicular lymphomas. Hum Pathol 2013;44:1817-26. [Crossref] [PubMed]
- Lin P, Dickason TJ, Fayad LE, et al. Prognostic value of MYC rearrangement in cases of B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and Burkitt lymphoma. Cancer 2012;118:1566-73. [Crossref] [PubMed]
- Campo E. Pathology and classification of aggressive mature B-cell lymphomas. Hematol Oncol 2017;35:80-3. [Crossref] [PubMed]
- Scott DW, Mottok A, Ennishi D, et al. Prognostic Significance of Diffuse Large B-Cell Lymphoma Cell of Origin Determined by Digital Gene Expression in Formalin-Fixed Paraffin-Embedded Tissue Biopsies. J Clin Oncol 2015;33:2848-56. [Crossref] [PubMed]
- Friedberg JW. How I treat double-hit lymphoma. Blood 2017;130:590-6. [Crossref] [PubMed]
- Raess PW, Moore SR, Cascio MJ, et al. MYC immunohistochemical and cytogenetic analysis are required for identification of clinically relevant aggressive B cell lymphoma subtypes. Leuk Lymphoma 2018;59:1391-8. [Crossref] [PubMed]
- Rosenthal A, Younes A. High grade B-cell lymphoma with rearrangements of MYC and BCL2 and/or BCL6: Double hit and triple hit lymphomas and double expressing lymphoma. Blood Rev 2017;31:37-42. [Crossref] [PubMed]
- Swerdlow SH. Diagnosis of 'double hit' diffuse large B-cell lymphoma and B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and Burkitt lymphoma: when and how, FISH versus IHC. Hematology Am Soc Hematol Educ Program 2014;2014:90-9. [Crossref] [PubMed]
- Miao Y, Hu S, Lu X, et al. Double-hit follicular lymphoma with MYC and BCL2 translocations: a study of 7 cases with a review of literature. Hum Pathol 2016;58:72-7. [Crossref] [PubMed]
- Miyaoka M, Kikuti YY, Carreras J, et al. Clinicopathological and genomic analysis of double-hit follicular lymphoma: comparison with high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements. Mod Pathol 2018;31:313-26. [Crossref] [PubMed]
- Aukema SM, van Pel R, Nagel I, et al. MYC expression and translocation analyses in low-grade and transformed follicular lymphoma. Histopathology 2017;71:960-71. [Crossref] [PubMed]
- Puvvada SD, Stiff PJ, Leblanc M, et al. Outcomes of MYC-associated lymphomas after R-CHOP with and without consolidative autologous stem cell transplant: subset analysis of randomized trial intergroup SWOG S9704. Br J Haematol 2016;174:686-91. [Crossref] [PubMed]
- Petrich AM, Gandhi M, Jovanovic B, et al. Impact of induction regimen and stem cell transplantation on outcomes in double-hit lymphoma: a multicenter retrospective analysis. Blood 2014;124:2354-61. [Crossref] [PubMed]
- Sun H, Savage KJ, Karsan A, et al. Outcome of Patients With Non-Hodgkin Lymphomas With Concurrent MYC and BCL2 Rearrangements Treated With CODOX-M/IVAC With Rituximab Followed by Hematopoietic Stem Cell Transplantation. Clin Lymphoma Myeloma Leuk 2015;15:341-8. [Crossref] [PubMed]
- Miyamoto K, Kobayashi Y, Maeshima AM, et al. Clinicopathological prognostic factors of 24 patients with B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and Burkitt lymphoma. Int J Hematol 2016;103:693-702. [Crossref] [PubMed]
- Bürgesser MV, Gualco G, Diller A, et al. Clinicopathological features of aggressive B-cell lymphomas including B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell and Burkitt lymphomas: a study of 44 patients from Argentina. Ann Diagn Pathol 2013;17:250-5. [Crossref] [PubMed]
- Havelange V, Ameye G, Theate I, et al. The peculiar 11q-gain/loss aberration reported in a subset of MYC-negative high-grade B-cell lymphomas can also occur in a MYC-rearranged lymphoma. Cancer Genet 2016;209:117-8. [Crossref] [PubMed]
- Brunn A, Nagel I, Montesinos-Rongen M, et al. Frequent triple-hit expression of MYC, BCL2, and BCL6 in primary lymphoma of the central nervous system and absence of a favorable MYC(low)BCL2 (low) subgroup may underlie the inferior prognosis as compared to systemic diffuse large B cell lymphomas. Acta Neuropathol 2013;126:603-5. [Crossref] [PubMed]
- Yuan J, Wright G, Rosenwald A, et al. Identification of Primary Mediastinal Large B-cell Lymphoma at Nonmediastinal Sites by Gene Expression Profiling. Am J Surg Pathol 2015;39:1322-30. [Crossref] [PubMed]
- Mottok A, Woolcock B, Chan FC, et al. Genomic Alterations in CIITA Are Frequent in Primary Mediastinal Large B Cell Lymphoma and Are Associated with Diminished MHC Class II Expression. Cell Rep 2015;13:1418-31. [Crossref] [PubMed]
- Steidl C, Gascoyne RD. The molecular pathogenesis of primary mediastinal large B-cell lymphoma. Blood 2011;118:2659-69. [Crossref] [PubMed]
- Gunawardana J, Chan FC, Telenius A, et al. Recurrent somatic mutations of PTPN1 in primary mediastinal B cell lymphoma and Hodgkin lymphoma. Nat Genet 2014;46:329-35. [Crossref] [PubMed]
- Jardin F, Pujals A, Pelletier L, et al. Recurrent mutations of the exportin 1 gene (XPO1) and their impact on selective inhibitor of nuclear export compounds sensitivity in primary mediastinal B-cell lymphoma. Am J Hematol 2016;91:923-30. [Crossref] [PubMed]
- Savage KJ, Monti S, Kutok JL, et al. The molecular signature of mediastinal large B-cell lymphoma differs from that of other diffuse large B-cell lymphomas and shares features with classical Hodgkin lymphoma. Blood 2003;102:3871-9. [Crossref] [PubMed]
- Chen BJ, Ruminy P, Roth CG, et al. Cyclin D1-positive Mediastinal Large B-Cell Lymphoma With Copy Number Gains of CCND1 Gene: A Study of 3 Cases With Nonmediastinal Disease. Am J Surg Pathol 2019;43:110-20. [Crossref] [PubMed]
- Morscio J, Dierickx D, Nijs J, et al. Clinicopathologic comparison of plasmablastic lymphoma in HIV-positive, immunocompetent, and posttransplant patients: single-center series of 25 cases and meta-analysis of 277 reported cases. Am J Surg Pathol 2014;38:875-86. [Crossref] [PubMed]
- Pan Z, Chen M, Zhang Q, et al. CD3-positive plasmablastic B-cell neoplasms: a diagnostic pitfall. Mod Pathol 2018;31:718-31. [Crossref] [PubMed]
- Valera A, Balague O, Colomo L, et al. IG/MYC rearrangements are the main cytogenetic alteration in plasmablastic lymphomas. Am J Surg Pathol 2010;34:1686-94. [PubMed]
- Ott G, Rosenwald A, Campo E. Understanding MYC-driven aggressive B-cell lymphomas: pathogenesis and classification. Blood 2013;122:3884-91. [Crossref] [PubMed]
- Vega F, Chang CC, Medeiros LJ, et al. Plasmablastic lymphomas and plasmablastic plasma cell myelomas have nearly identical immunophenotypic profiles. Mod Pathol 2005;18:806-15. [Crossref] [PubMed]
- Nicolae A, Pittaluga S, Abdullah S, et al. EBV-positive large B-cell lymphomas in young patients: a nodal lymphoma with evidence for a tolerogenic immune environment. Blood 2015;126:863-72. [Crossref] [PubMed]
- Hofscheier A, Ponciano A, Bonzheim I, et al. Geographic variation in the prevalence of Epstein-Barr virus-positive diffuse large B-cell lymphoma of the elderly: a comparative analysis of a Mexican and a German population. Mod Pathol 2011;24:1046-54. [Crossref] [PubMed]
- Adam P, Bonzheim I, Fend F, et al. Epstein-Barr virus-positive diffuse large B-cell lymphomas of the elderly. Adv Anat Pathol 2011;18:349-55. [Crossref] [PubMed]
- Yoon H, Park S, Ju H, et al. Integrated copy number and gene expression profiling analysis of Epstein-Barr virus-positive diffuse large B-cell lymphoma. Genes Chromosomes Cancer 2015;54:383-96. [Crossref] [PubMed]
Cite this article as: Chen BJ, Fend F, Campo E, Quintanilla-Martinez L. Aggressive B-cell lymphomas—from morphology to molecular pathogenesis. Ann Lymphoma 2019;3:1.


