
Is there a role for immunomodulatory drugs in the treatment of mantle cell lymphoma?
Introduction
Immunomodulatory drugs (IMiDs) refer to thalidomide and its derivatives. To date, three agents are available for use, thalidomide, pomalidomide and lenalidomide (CC-5013) of which lenalidomide is the one with approval status by US Food and Drug Administration (FDA) and European Medicines Agency (EMA) for use in mantle cell lymphoma (MCL) (1,2). Furthermore, a number of novel immunomodulatory agents, such as CC-122, CC-223 and CC-292, are currently evaluated in phase I trials in patients with chronic lymphocytic leukemia (CLL), and non-Hodgkin lymphoma (NHL). This overview aims to discuss the role of lenalidomide in MCL based on reported data as well as to provide a glance of how ongoing studies and future analysis will clarify the optimal use of these agents in MCL.
IMiDs exhibit a spectrum of anti-tumoral effects
IMiDs are associated with several properties, including direct anti-proliferative and anti-angiogenic effects on tumor clones as well as immunomodulatory actions associated with enhanced anti-tumoral activity mediated by the host immune system (Figure 1). Although mostly studied in multiple myeloma (MM), mechanisms of action (MOA) have been confirmed in in vitro and in vivo models on B cell lymphoma (3-5). The immune activating capacity of IMiDs could be regarded as the most important and reproducible mechanism, as demonstrated by activated dendritic cells (DC) and increased secretion of interleukin 2 (IL-2) leading to recruitment and activation of T cells, natural killer (NK) cells and activated pro-inflammatory response by secretion of cytokines, such as tumor necrosis factor α (TNF-α), interferon gamma (INFγ) and monocyte chemotactic protein-1 (MCP-1) (3). A fundamental point in all these actions is the intracellular binding to cereblon (CRBN), which is involved in the formation of a ubiquitination ligase complex (6). Treatment with lenalidomide has been demonstrated to induce ubiquitination and degradation of two transcription factors, Ikaros and Aiolos (IKCZ1 and IKCZ3), and thereby altering the expression of several genes involved in proliferation and activity of B and T cells, such as increased levels of p21 and reduced expression of MYC proto-oncogene protein (MYC), Sp1 gene B (SP1B) and interferon regulatory factor 4 (IRF4) proteins (7,8). Degradation of IKCZ1 and IKCZ3 has also been correlated with increased IL-2 secretion by T cells, increased levels of CD8+ cytotoxic T cells, decreased levels of T regulatory cells and recruitment and activation of NK cells, altogether representing an activation of the immune response (3,8).
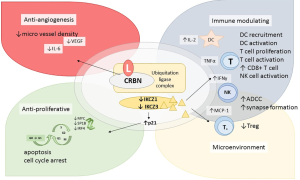
Furthermore, IMiDs have demonstrated a capacity to overcome tumor-related evasion, by restoring the reduced immunologic synapse formation between the T cell and the tumor cell, as observed in in in vitro assays on follicular lymphoma (FL) and CLL (9,10).
The anti-angiogenic effects have been described as reduced micro vessel density and may be explained by depletion of macrophages and monocytes involved in lymphoma-related angiogenesis (3,11).
IMiDs may synergize with dexamethasone and/or anti-CD20 antibody treatment
A synergistic activity of lenalidomide and dexamethasone in MCL cell lines and patient-derived MCL cells was demonstrated by Qian et al. with higher grade of cell cycle arrest, higher expression of pro-apoptotic proteins such as caspases, Bcl-2 and Bax, higher rate of apoptotic cells and decreased tumor growth upon combination of these two agents (12).
Targeting CD20 by a monoclonal anti-CD20 antibody, i.e., rituximab, has been proved to be highly active in MCL and constitutes a backbone of primary treatment. Upfront addition of rituximab to chemotherapy, is associated with superior survival in retrospective analysis of large population-based cohorts and randomized trials have demonstrated prolonged disease control and improved survival when given as maintenance after immunochemotherapy (13-16).
The MOA of anti-CD20 antibody treatment include complement-dependent cytotoxicity (CDC), antibody-dependent cellular cytotoxicity (ADCC) and direct cell death via apoptosis, of which ADCC, mediated by different effector cells of the immune system, is regarded as one of the more important in the activity in NHL and MCL as reviewed by Boross et al. (17).
Based on the enhanced T cell activity upon treatment with lenalidomide, in vitro models have investigated whether lenalidomide could increase the immune-mediated cytotoxicity induced by monoclonal anti-CD20 antibody treatment. A first report in 2005 described a tendency of synergistic effect of CC-5013 (lenalidomide) when combined with rituximab in vitro (18). Wu et al. demonstrated a synergistic effect on NK-cell mediated cell killing by pre-treatment of NK cells with lenalidomide, with increased cytotoxicity of anti-CD20 coated lymphoma cells and increased release of IFNγ (19). Similar studies confirmed the enhanced NK cell activity and ADCC by combination of immune modulators including lenalidomide and rituximab in B cell lymphoma and MCL in vitro and ex vivo models, as shown by increased recruitment of NK cells, increased levels of IL-2 and increased expression of CD16 and IFNγ in NK cells (3,20).
Lenalidomide in MCL
Single agent may induce response in R/R MCL
The initial trials evaluating single agent lenalidomide, NHL-002 and NHL-003, were designed for NHL and MCL patients constituted a subgroup of the study population. Both trials used 25 mg daily for 12 months treatment (NHL-002) or until PD (NHL-003) and primary endpoints were overall response rate (ORR). Based on the promising activity in the subgroups of MCL patients demonstrating ORR of 47% and 53% respectively (Table 1), trials designed for MCL patients were initiated (21,38).
Table 1
| Regimen | Ref | Trial | Phase | Diagnosis | Dose L* | n MCL | Dose L in M | ORR (CRR) | md DOR | md FU time | PFS | OS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Single agent lenalidomide | (21,22) | NHL-002 | II | R/R NHL | 25 mg | 15 (49 tot) | 53% (20%) | 14 m (4 NR) | NR | md 6 m | ||
| (23) | UK (Eve) | II | R/R MCL | 25 mg | 26 | 15 mg | 31% (8%) | 22 m | 23m | ITT: md 4 m; M: md 15 m | md 10 m | |
| (24) | NHL-003 | II | R/R aggr. NHL | 25 mg | 57 (217 tot) | 53% (20%) | 16 m (7 NR) | Resp pts 20 m | ||||
| (25,26) | MCL-001 (EMERGE) | II | R/R MCL post-BOR | 134 | 28% (8%) | 17 m | 10 m | md 4 m | md 19 m | |||
| L vs. investigator’s choice | (27) | MCL-002 (SPRINT) | III, rand | R/R MCL | 25 mg | 170 | 68% (40%) | 16 m | 41 m | md 9 m | md 28 m | |
| Inv.’s choice | 84 | 9% (11%) | 10 m | md 5 m | md 21 m | |||||||
| L + dexamethasone | (28) | II | R/R MCL | 33 | 52% (24%) | 18 m | md 12 m | md 20 m | ||||
| L + rituximab (R2) | (29) | NCT 00294632 | I/II | R/R MCL | MTD: 20 mg | 44 | 57% (36%) | md 18.9 m (17.0 NR) | 23 m | md 11 m | md 24 m | |
| (30,31) | II | Untreated MCL | I: 20 mg c 1, 25 mg c 2–12; R† | 38 | 15 mg, R†/8 w 3 y | 61% | – | 68 m | 3-y PFS 80.3%; 5-y PFS 64% | 3-y OS 92%; 5-y OS 77% | ||
| L + obinutuzumab | (32) | GALEN | II | R/R aggr. NHL | 20 mg, O 1,000 mg; d 8, 15, 22 c 1, d 1 c2–6, 1/8 w during M | 13 (85 tot) | – | 39% (23%) | md NR | 2.5 y | md 5.8 m | NR |
| R2-bendamustine | (33) | R2-FIL | II | R/R MCL | MTD: 10 mg d 1–14/28 d, c 1–4; 15 mg d 1–14/28 d, c 5–6 | 42 | 15 mg d 1–14/28, c 7–18 | Of evaluated 4 c: 88% (44%); 6 c: 79% (58%) | – | 29 m | md 20 m | 2-y OS 67% |
| (34) | MCL4 (LENA-BERIT) | I/II | Untreated MCL ≥65 y | MTD: 10 mg, c 1–6; 10 [15] mg, c 7–13 | 50 | M L ×7 | ITT 6 c: 80% (64%) | – | 45 m | md 42 m | md 69 m | |
| R2-bortezomib | (35) | NCT 00633594 | I/II | Untreated or R/R MCL | MTD: 10 mg d 1–14//21 d BOR‡ | 22 | Of evaluated 82% (32%) | – | 16 m | 18 m PFS 61% | 18 m OS 79% | |
| (36) | CALBG 5051 | II | R/R MCL | 20 mg d 1–14/21 d, BOR, R† ×8 | 53 | 40% (15%) | – | 46 m | md 7 m | md 26 m | ||
| R2-ibrutinib | (37) | MCL6 (PHILEMON) | I + II | R/R MCL | L 15 mg | 50 | I + R | 76% (56%) | – | 18 m | 12-m PFS 57% | 12 m OS 78% |
*, d 1–21, cycle duration 28 d; †, R 375 mg/m2 iv. 1/week c 1; 1/8 w c 2- (d 1 each cycle if with chemotherapy); ‡, BOR 1.3 mg/m2 iv. d 1, 4, 8, 11. ITT, intention to treat; NR, not reached; NCT, trial number at
In the MCL-001 trial, 134 patients who relapsed after prior treatment with bortezomib received lenalidomide 25 mg daily until progression. At a median follow-up (FU) time of 9.9 months, 28% had responded with a median duration of response (DOR) time of 16.6 months (95% CI, 7.7 to 26.7 months) (26). Another trial, reported by Eve et al., evaluated 6 cycles of lenalidomide, 25 mg daily, followed by maintenance lenalidomide 15 mg daily, in 29 included patients, demonstrating 31% ORR, and a median DOR of 22 months (23).
These results lead to US FDA approval of lenalidomide to patients with MCL who had relapsed after treatment with bortezomib (39). A subsequent randomized phase III trial (MCL-002; SPRINT) compared lenalidomide with investigator’s choice in 292 patients (2:1 lenalidomide: investigator’s choice) which demonstrated a benefit in the lenalidomide group leading to approval in Europe by EMA (2). Objective response rate in the lenalidomide group was 40% and median progression-free survival (PFS) was 8.7 months, compared to 11% and 5.2 months in the investigator’s choice group. Although the median DOR was longer in the lenalidomide group, no significant difference in overall survival between the groups could be demonstrated (27,40).
A long term analysis on 206 MCL patients included in the initial trials NHL-002, NHL-003 and MCL-001 after a median FU time of 6.8 years, 7.6 years and 52.2 months respectively, demonstrated a best ORR of 33%, complete remission rate (CRR) 11% and a median DOR of 16.6 months (22).
Lenalidomide in combination with dexamethasone and anti-CD20 antibody
Based on the preclinical demonstration of increased cell cycle arrest and apoptotic activity by combination of lenalidomide and dexamethasone, this regimen was evaluated in a phase II trial, demonstrating 52% ORR in 33 patients with MCL (12,28).
Given the enhanced immune activity by lenalidomide and an anti-CD20 antibody in vitro, the combination of rituximab and lenalidomide (R2) was explored in clinical trials. Wang et al. reported data from the initial phase I/II trial on R/R MCL patients, combining rituximab and lenalidomide, with somewhat higher response rates; ORR 57% and CRR 36%, compared to single agent lenalidomide. All patients had previously received rituximab, of which nine patients had received rituximab as maintenance therapy and eight patients as part of the latest regimen (29,41).
Chong et al. investigated the potency of lenalidomide to re-sensitize previously rituximab resistant/refractory disease. Fifty patients with B cell lymphoma, previously defined as resistant/refractory to rituximab, received two cycles of pre-treatment with lenalidomide followed by the addition of rituximab, with observed doubling of ORR from 30% to 63%. Of note, in the subgroup of MCL patients (n=11), ORR (55%) after single lenalidomide did not increase by the addition of rituximab, albeit some more patients achieved complete remission (Table 1) (42).
R2 has been investigated as an upfront “chemo-free” regimen in one phase II trial reported by Ruan et al. (30,31). In the trial cohort, a majority of patients (68%) were scored as low or intermediate-risk according to mantle cell lymphoma prognostic index (MIPI), 79% of the cases were scored with Ki-67 ≤30% and no one were blastoid MCL. Patients with high-risk MIPI were only included in case of ineligibility to tolerate chemotherapy. Of 36 evaluable patients, 61% achieved complete remission and according to a recent update at a median FU time of 64 months, survival analysis demonstrated a 5-year PFS of 64% and 5-year OS of 77%.
Lenalidomide has also been combined with the type II anti-CD20 antibody obinutuzumab within the GALEN trial, by the French LYSA network, to R/R FL and aggressive B cell lymphoma. In the latter cohort, ORR (CRR) was 39% (23%) in the subgroup of 13 MCL patients of which 53% had rituximab-refractory disease (32).
Lenalidomide and immunochemotherapy
Primary treatment of MCL include immunochemotherapy and for young fit patients, consolidation with high dose chemotherapy with autologous stem cell support (HD-ASCT). Elderly patients are recommended rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) followed by maintenance rituximab or R-bendamustine, the latter associated with a preferable toxicity profile compared to R-CHOP as reported from one randomized trial by the German STiL group (43). Consequently, R-B was used to explore lenalidomide in combination with immunochemotherapy in two trials, the Nordic MCL4 (LENA-BERIT) to untreated elderly patients ≥65 years, and the Italian trial FIL-R2-B to R/R MCL patients. In the cohort of 50 patients in MCL4, ORR (CRR) was 80% (64%) after induction with R-B-L ×6 and 56% were negative in evaluation of minimal residual disease (MRD), not increased by maintenance treatment with lenalidomide (34). In FIL-R2-B, reported by Zaja et al., ORR (CRR) was 79% (55%) in 42 patients after induction with R-B-L ×4 followed by consolidation with two cycles of R2 (33). Out of responding patients, 36% achieved MRD negativity in bone marrow (BM). These results suggest that the addition of lenalidomide to R-B is an active regimen and a substantial portion of the patients may achieve MRD negativity. As discussed in following sections, toxicity was unexpectedly high in the untreated cohort and patients with high-risk profile like TP53 mutations may not profit from this combination (44).
R-L-CHOP to untreated DLBCL patients has been proved to be more tolerable, possibly due to the corticosteroids included in the regimen (45). Two trials are currently evaluating the addition of lenalidomide to anthracycline-based immunochemotherapy regimens in MCL, the Memorial Sloan Kettering (NCT02633137) including R-L-CHOP followed by R-HiDAC (high-dose cytarabine) and maintenance R2, and the randomized European R2 Elderly trial evaluating the addition of lenalidomide to R-CHOP as well as maintenance R2 compared to rituximab (NCT01865110) (Table 2) (46).
Table 2
| Trial/site | NCT number | Phase | Inclusion | Regimen |
|---|---|---|---|---|
| R2-venetoclax | ||||
| NLG-MCL7 (VALERIA) | NCT03505944 | I/II | R/R MCL or untreated MCL* | |
| University of Michigan Cancer Center | NCT03523975 | I | Untreated MCL | |
| R2-ibrutinib | ||||
| MD Anderson | NCT03232307 | I/II | Untreated MCL | |
| R2-carfilzomib | ||||
| MD Anderson | NCT01729104 | I/II | R/R MCL | |
| L-R-chemo + L-R maintenance | ||||
| Memorial Sloan Kettering Cancer Center | NCT02633137 | II | Untreated MCL | R2-CHOP ×4 → R-HiDAC→ R2 ×6 |
| L maintenance | ||||
| European MCL-R2 Elderly | NCT01865110 | III, rand | Untreated MCL | R-CHOP/R-HAD ×6 vs. R-CHOP ×8; maintenance R2 vs. R |
| MAGNIFY | NCT01996865 | III, rand | R/R MCL* | R vs. L maintenance |
| FIL MCL0208 | NCT02354313 | III, rand | Untreated MCL | R-chemo + ASCT → L vs. observation |
| EOCG randomized | NCT01415752 | II, rand, 4-arm | Untreated MCL | R-B vs. R-B-BOR followed by maintenance R2 vs. R |
| L + BiTE-ab | NCT02568553 | I | CD19 pos R/R lymphoma | L-blinatumomab |
| Closed trials (cause of termination) | ||||
| RENEW (not relevant) | NCT01021423 | III, rand | Untreated MCL | R-CHEMO → L maintenance vs. observation |
| R-LEN-idelalisib (DLT) | NCT01088048 | I/II | DLT |
NCT, trial number at
Lenalidomide in chemo-free regimens
Several trials have investigated further development of R2 by addition of a third novel agent such as a small molecule inhibitor. In this overview, we prefer to include reported data from combinations with the proteasome inhibitor bortezomib, the inhibitor of Bruton’s Tyrosine Kinase (BTK) ibrutinib, and the phosphoinositide 3 kinase (PI3K) inhibitor idelalisib. Moreover, registered/ongoing trials evaluate combinations with the novel proteasome inhibitor carfilzomib, the bcl-2 inhibitor venetoclax as well as substituting rituximab with a type II anti-CD20 antibody, obinutuzumab (Table 2).
Bortezomib was approved by FDA for treatment of R/R MCL in 2006 and as part of first-line therapy in 2014 based on superiority of addition of bortezomib to anthracycline-based immunochemotherapy (R-CAP vs. R-CHOP) (1,47,48). Lenalidomide and bortezomib was explored in a phase I/II trial on 54 R/R MCL patients, showing an ORR (CRR) of 40% (15%) (NCT00553644) (36). One registered trial, SCRI LYM 58, is investigating R-L-bortezomib in R/R or untreated MCL (NCT 00633594). The second-generation proteasome inhibitor carfilzomib, developed to increase response duration and to diminish sensory neuropathy associated with bortezomib, has been combined with L + dexamethasone with acceptable tolerability in MM (49). Although not yet reported as single agent in MCL (phase II trial NCT 02042950 is ongoing), a phase I/II trial conducted by M.D. Anderson Cancer Center, US, will evaluate R2 + carfilzomib to R/R MCL patients (NCT01729104).
Ibrutinib has showed high activity in MCL and has achieved a position of “drug of choice” at first relapse in current guidelines (50). Ibrutinib + R2 (R-L-I) was evaluated within the Nordic MCL6 (PHILEMON) trial in R/R MCL, where patients received twelve months of R-L-I followed by maintenance ibrutinib + rituximab (IR). At median FU time of 17.8 months ORR was 76% and CRR 56% (37). Of note, response was observed even in TP53 mutated cases, otherwise associated with poor prognosis. Besides another trial in R/R MCL (NCT02446236), potentially confirming the activity of this combination, ibrutinib-R2 will be evaluated in a phase II trial in untreated patients >65 years (NCT03232307).
Less successful outcome was observed when the PI3K inhibitor idelalisib was combined with rituximab and lenalidomide. Two trials have reported unexpected severe toxicity including sepsis, hypotension, rash and impaired liver function when these agents were combined (51,52).
Among recently introduced compounds, the bcl-2 inhibitor venetoclax (ABT-199) may be one of the most promising agents, demonstrating overall response in >70% in R/R MCL patients, previously treated with a median number of 3 [1–7] prior therapies and a potency of durable remission in responding patients (53). Two phase I/II trials are registered for the evaluation of venetoclax in combination with rituximab and lenalidomide, the Nordic MCL7/VALERIA (NCT03505944) in R/R MCL or untreated (not eligible for chemotherapy) and a phase I trial for previously untreated MCL patients (NCT0352975).
Maintenance treatment with lenalidomide
According to reported data, maintenance treatment in MCL may principally have a role for a subset of patients, although further studies are required to identify the specific patient population as well as to define which regimen that should be used. To date, our knowledge is based on single arm trials and a few randomized trials, where rituximab maintenance after immunochemotherapy has been associated with prolonged response duration and superior survival as recently reviewed after meta-analysis by Hilal et al. (54). Maintenance lenalidomide has been administered after single agent lenalidomide in an initial R/R MCL trial, in combination with rituximab for maximally three years after upfront R2, and after R-B-L in the MCL4 and FIL-R2B (23,30,34,55).
In spite of different protocol length of induction and maintenance phase in these trials, the results indicate that maintenance lenalidomide may increase CRR in responding patients, albeit to a limited extent. Ruan et al. reported a CRR of almost 60% at 24 months after start of treatment, compared to 45% at 12 months in evaluated patients who received maintenance with R2. Still, the majority of complete remissions were found during the early treatment phase as demonstrated by a reported median time [range] to response and complete remission of 3 [2–13] and 11 [3–22] months respectively (30). In the Italian trial FIL-R2B, CRR increased from 44% to 55% after consolidation with 2 cycles of R2 after R-B-L ×4, although MRD negativity in BM did not increase. Moreover, subsequent single agent lenalidomide up to 18 months after R-B-L as in FIL-R2B did not maintain disease control and the PFS curve did not show a plateau (33). A similar pattern was observed after R-B-L in MCL4, where molecular remission in BM was achieved in 42% of all patients, not improved by maintenance treatment with lenalidomide (34).
A relevant point in evaluating a maintenance regimen, besides response and survival, is the toxicity profile with respect to duration of the treatment and quality of life. According to reported data, the risk of grade ≥3 adverse events is not eradicated by lowering of lenalidomide dose from 20 mg to 15 mg and several trials report a sustained BM suppression during maintenance with lenalidomide after induction with regimens including anti-CD20 antibody with or without chemotherapy, as demonstrated in the R-B-L trials FIL-R2B and MCL4.
In summary, prolonged treatment with lenalidomide ± rituximab may be relevant in a small portion of patients but available data is not sufficient to conclude whether the benefit is explained by lenalidomide or by the anti-CD20 component. Current randomized trials will hopefully lead to a better understanding of its potency to consolidate and sustain remission, either as single agent as in the phase III trial MAGNIFY (NCT01996865) to R/R MCL or in combination with rituximab in the EU Network trial MCL R2 Elderly to untreated MCL (NCT01865110) (Table 2).
Toxicity profile of lenalidomide in MCL patients
Myelosuppression and risk of infection
Witzig et al. performed a pooled analysis of grade 3/4 adverse events in the initial trials, NHL-001, -002-003, MCL-001 and a UK phase II trial including all patients and not only MCL (56). Grade 3/4 neutropenia, thrombocytopenia and anemia were the most common side effects, reported in 42%, 28% and 11% respectively. Similar frequencies could be observed when lenalidomide was combined with rituximab or dexamethasone (29,30,55). Of note, in spite of a high frequency of grade 3 neutropenia among the patients, grade 3/4 febrile neutropenia or infection were reported in less than 10% of the study population.
However, when lenalidomide is combined with chemotherapy, the safety profile is markedly different, especially in previously untreated patients. The MCL4 trial reported grade 3/4 neutropenia in 76% at any time during induction with R-B-L or maintenance L. Grade ≥3 infection was reported in 42% of the patients, significantly higher compared to the R-B-L in R/R patients in the SAKK and FIL-R2B trials respectively, in spite of comparable rate of high grade neutropenia in the three trials (33,34,57). The mechanism behind the observed higher risk of infection in untreated patients remains to be clarified but might be explained by difference in immune activity compared to when being previously exposed for immunochemotherapy. Moreover, in the trial of untreated patients in MCL4, three patients were diagnosed with opportunistic infections, two with pneumocystis pneumonia (PCP) and one patient with CMV-retinitis, reflecting a more profound immunosuppression when lenalidomide is combined with a T-cell suppressive agent such as bendamustine and a CD20 ab.
In MCL6, grade 3/4 neutropenia and infection were observed in 38% and 22% respectively in patients treated with R-L-I, indicating that the risk of myelosuppression is not eradicated by chemo-free strategies (37).
In summary, lenalidomide is associated with hematologic toxicity and an elevated risk for severe infections has been observed in treatment-naïve patients or when lenalidomide is combined with other cytostatic compounds or small molecule inhibitors.
Immune-related side effects include rash and allergic reactions
Among non-hematologic side effects of lenalidomide, the immune-related toxicity including cutaneous and allergic reactions should be emphasized. Rash grade ≥3 was reported in less than 10% of patients with R/R disease receiving lenalidomide as monotherapy or in combination with rituximab or dexamethasone (56). Severe cutaneous reactions, are more frequent in treatment-naïve patients, as reported in 23% with R2, 32% with R2-bortezomib and 18% in the R-B-L compared to <10% in previously treated patients or patients receiving lenalidomide monotherapy (30,34,35,56). R-CHOP + lenalidomide was investigated as first line treatment to patients with diffuse large B cell lymphoma (DLBCL) but did almost not report any rash (2%). The low frequency could be explained by a prophylactic or hampering effect by the administration of corticosteroids within the regimen, similarly to the reduction of early reactions after protocol amendment in MCL4 phase I portion by adding corticosteroids to first cycles of L-R-B (34,45).
Lenalidomide and thalidomide are associated with increased risk of venous thromboembolic events (VTE). Although previous studied is mostly based on MM as reviewed by Carrier in 2011, a recent systemic meta-analysis has stated that NHL patients are at the same risk as myeloma patients upon treatment with these agents, although possibly lower by combination of L with biologics compared to L + chemotherapy (58,59). To date, there is no consensus whether all patients should receive VTE prophylaxis during treatment but should be carefully evaluated in each individual case (2).
Altogether, these data indicate that the toxicity profile of lenalidomide, either as monotherapy or in combination with dexamethasone or rituximab, is acceptable, especially in previously treated patients. However, treatment-naïve patients may be more susceptible to severe toxicity upon treatment with lenalidomide in combination with rituximab and the addition of a third partner, such as bendamustine and bortezomib, is associated with a higher risk of dose-limiting toxicity.
Second primary malignancy (SPM) is a late onset complication of immunomodulatory agents
Lenalidomide is associated with increased risk of SPM. A higher frequency of SPMs was demonstrated in a pooled analysis of patients with MM treated with lenalidomide within randomized trials and a higher incidence of SPMs was reported in MM patients treated with lenalidomide compared to a population-based age-adjusted incidence (60-63).
MCL patients have been suggested to be at a higher risk of developing SPMs, compared to the general population, as demonstrated from a large population-based analysis by Shah et al. (64). Concerning the incidence of SPMs in MCL after treatment with lenalidomide upfront, Ruan et al. reported SPMs in 6 of 38 (16%) patients receiving R-lenalidomide at 5 years median FU time in SPM was reported in 18% of the patients at a median FU of 4 years (31,44).
There is no established theory to explain the increased risk of a SPM. Binding of lenalidomide to cereblon have been associated with deregulated cell cycle regulation via p21, CDK/cyclin complex and p53 as well as via IKZF1+3 ubiquitination mechanisms (6,8). With that in mind, one could speculate whether the cellular repair systems for DNA damage induced by an alkylating agent such as bendamustine are inhibited by concomitant use of lenalidomide and thereby put the cell less resistant to stress and genetic alterations.
However, as Demopoulos discussed in MM patients, the risk of SPMs in MCL patients should be weighed towards the potential benefit of lenalidomide treatment. We recommend that patients with a history of a prior malignancy should be carefully evaluated for active/pre-stage malignancy before and during treatment of lenalidomide.
Who will respond to lenalidomide based on tumor characteristics?
Although several prognostic factors have been described in MCL, such as morphologic subtype, proliferation rate of the tumor cells as well as presence of specific genetic alterations, few trials incorporate analysis of outcome in relation to a broad panel of base-line prognostic markers.
The proliferation marker Ki-67 is one of the most established prognostic factors in MCL and evaluation of Ki-67 is recommended to be included in routine diagnostic (50). In patients treated with single agent lenalidomide within the MCL-001 trial, Ki-67 >30% or >50% respectively was associated with inferior PFS and OS, leading to the conclusion that single agent lenalidomide is not active in previously bortezomib-treated R/R MCL with high Ki-67% (25).
Recent studies on prognostic factors in MCL have demonstrated a correlation of prognosis and presence of specific genetic alterations of the tumor cells, of which deletions and/or mutations of CDKN2A and/or TP53 have been associated with inferior outcome after standard treatment with immune chemotherapy (65-67). In untreated patients within the MCL4 trial, patients harboring TP53 mutations were associated with inferior PFS and OS compared to non-mutated cases and none of these patients achieved MRD (44). However, when lenalidomide was combined with rituximab and ibrutinib in the MCL6 trial, response was observed in TP53-mutated cases, which is similar as observed from a trial in CLL patients where lenalidomide was associated with activity in TP53-aberrated patients (37,68). Still, it remains to be clarified if and in which combination lenalidomide is capable of eradicating MCL clones with non-functional p53.
In summary, studies on activity of IMiDs in relation to molecular profile are few. So far, data suggests that single agent lenalidomide is not sufficient in patients with highly proliferative MCL and probably not in cases with poor molecular profile, although an approach using lenalidomide in combination with multiple agents may be more active.
Discussion
Although historically associated with its teratogenic effect, causing one of the worst tragedies in pharmacology history, thalidomide has been re-explored based on its immune modulating and anti-tumoral activity. Thalidomide and further development of the molecule, referred to as immunomodulatory agents, IMiDs, have demonstrated activity in both solid tumors and hematologic malignancies. Lenalidomide is the most studied immunomodulatory agent in MCL and is thus the focus of this review. In this section we aim to discuss and give our perspectives on how to use IMiDs in MCL based on reported data and personal experience.
One of the most interesting properties of IMiDs is the potency of enhancing the immune response to monoclonal antibody treatment, including anti-CD20 antibody, which today constitutes the backbone of MCL treatment. Based on reported data from a few single arm trials in R/R MCL, one could argue that R-L is preferable, as demonstrated by higher CRR compared to L monotherapy. In patients with rituximab-refractory disease, there is not enough support of a re-sensitizing effect of L in MCL as reported from other NHL. In these patients, L + obinutuzumab might be a better option, although reported data needs to be confirmed from larger cohorts.
According to population-based analyses of MCL, one group in need of improved treatment outcome is the aged patient population, partly due to limited treatment options with respect to efficacy and tolerability (16). Given the limited toxicity when L is combined with anti-CD20 antibody and the results from the trial on R2 in first-line reported by Ruan et al., this combination might be a preferable option in elderly patients with low/intermediate risk disease according to MIPI, alternatively in frail patients non-eligible for chemotherapy (31). A randomized trial would possibly confirm whether lenalidomide is superior to established regimens in these patients.
The efforts to reinforce induction with immunochemotherapy by addition of lenalidomide have reported somewhat conflicting results. Although a deep remission may be achieved with R-B + lenalidomide, toxicity has been dose-limiting, especially in the untreated patient population. Lenalidomide + R-CHOP may be more tolerable but there is not sufficient data to evaluate this regimen in MCL (69). Future trials, such as the randomized MCL R2 Elderly will bring further insight whether patients do benefit from the addition of lenalidomide to standard immunochemotherapy.
Out of experiences from lenalidomide in chemo-free regimens, R-L-I is an active and tolerable combination with potency of achieving deep remissions with undetectable MRD in R/R MCL patients (37). One can speculate whether L contributes to a deeper remission, based on the observed higher CRR in R-L-I (MCL6 trial) compared to R-I (37,70). Notably, ORR does not seem to be higher by R-L-I, suggesting that lenalidomide may only have an additive effect in responding patients. Evaluation of MRD after treatment with these agents on an intention-to treat basis is needed to confirm an additive effect of lenalidomide. Moreover, trials reported on R + I and R + L in R/R or untreated MCL do not report outcome in relation to poor prognostic markers such as persisting MRD positivity or TP53 alterations why it is still unclear about whether lenalidomide has any additive affect in these cases.
As described in previous sections, toxicity may be an issue in patients receiving IMiDs. With lenalidomide, the most relevant side effects include immune mediated reactions, rash, neutropenia with increased risk of infection, venous thromboembolism and SPM which should be carefully evaluated by the responsible clinician before and during treatment with lenalidomide.
Our experience from the Nordic phase II trials MCL4 and MCL6 is that immune-mediated reactions are more likely to appear in treatment-naïve patients, and when IMiDs are combined with other agents, which should be taken into account when designing trial protocols involving novel regimens. Adverse reactions could be minimized by stepwise introduction of L, eventually with concomitant use of corticosteroids during the first [1–2] cycles, as used in an MD Anderson trial (NCT03232307) on R-L-I to untreated MCL. When using single agent L in R/R MCL, the risk is negligible and patients can generally start at the recommended dose of L 25 mg.
Grade 1–2 rash could generally be managed with local corticosteroids, without treatment discontinuation whereas grade 3 rash requires temporarily stopped administration of L and 1–2 weeks of systemic corticosteroids and anti-histamine before L could be re-started. Rash is not diminished or prevented by a lower dose of L, although it may be appropriate to re-start at a lower dose in patients with a history of grade 3 rash with subsequent dose-escalation if tolerated.
Mild neutropenia could generally be managed by dose reduction, but in case of neutrophil count below 103/µL, L should be temporarily stopped until recovering.
In patients receiving single agent L, we do not routinely prescribe VTE-prophylaxis. However, when combined with other agents, or in patients with a history of VTE, prophylaxis is used.
Similarly, prophylactic antibiotics cannot generally be recommended. As discussed in the previous section, PCP-prophylaxis and G-CSF should be used routinely in case of persisting myelosuppression, recurrent use of corticosteroids and when L is combined with other myelosuppressive agents.
Finally, we would like to advise against using IMiDs in patients that have previously received allogenic stem cell transplant, as this may provoke a severe and potentially fatal graft-versus-host reaction (71).
In R/R MCL, the BTK-inhibitor ibrutinib has shown high response rates, and besides being recommended at first relapse in current guidelines, ibrutinib is evaluated as part of first line treatment within several ongoing trials in elderly and young patients with MCL (50). Concerning the activity of lenalidomide in relation to ibrutinib, it should be noted that the SPRINT trial comparing L to investigator’s choice was performed prior to the introduction of ibrutinib. Moreover, resistance to ibrutinib, either primary or acquired is a well-described phenomenon and has been associated with short overall survival in pooled retrospective analysis (72). One study has investigated outcome of lenalidomide-regimens in patients with R/R MCL post-ibrutinib, demonstrating an ORR of 28%, the majority of responding cases in patients receiving lenalidomide in combinatory regimens.
Consequently, as future patients will have received a BTK inhibitor, either at first relapse or upfront within any of the ongoing clinical trials, patients may be treated with R + L at relapse, preferably in combination with a third agent.
Given that IMiDs have a place in treatment of MCL, there are two main points that should be considered in designing future trials to provide a more economic use of these compounds; to whom and for how long time IMiDs should be administered. To date, trial protocols have included IMiDs with or without rituximab for long time periods, such as three years or until progression. Individual evaluation of discontinuous administration, i.e., at CR or MRD negativity, would not only reduce long-term toxicity but also the economic burden in public health systems. Similarly, outcome in relation to specific patient or disease characteristics, such as p53 expression and/or TP53 alterations, Ki-67% as well as MIPI should be integrated into trial design in order to identify which patients that do benefit from immunomodulatory agents.
In summary, lenalidomide may have a place in MCL upfront, in combination with anti-CD20 ab in low-risk elderly patients. Likewise, R/R MCL patients may benefit from lenalidomide, preferably in combination with multiple agents. The outcome of several ongoing trials will reveal if lenalidomide should be added upfront to immunochemotherapy regimens or be a part of a chemo-free strategy. Similarly, exploration of novel regimens including IMiDs in combination with novel small molecule inhibitors such as BTK-inhibitors, venetoclax, second generation anti-CD20 antibodies, bi-specific antibodies or chimeric antigen receptor (CAR) T cells, will hopefully lead to a better understanding of how these agents optimally could be used and how to improve outcome in patients with MCL.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Martin Dreyling) for the series “Future Directions for Mantle Cell Lymphoma” published in Annals of Lymphoma. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aol.2019.01.01). The series “Future Directions for Mantle Cell Lymphoma” was commissioned by the editorial office without any funding or sponsorship. M Jerkeman has received honoraria and financial support for clinical trials from Celgene, Janssen, Abbvie, Gilead and Roche. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
-
U.S. Food and Drug Administration 2018 . Available online: www.fda.gov - European Medicines Agency. Revlimid-Summary of Product Characteristics. European Medicines Agency. www.ema.europa.eu. 2018. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000717/WC500056018.pdf. Accessed 23 Aug 2018.
- Reddy N, Hernandez-Ilizaliturri FJ, Deeb G, et al. Immunomodulatory drugs stimulate natural killer-cell function, alter cytokine production by dendritic cells, and inhibit angiogenesis enhancing the anti-tumour activity of rituximab in vivo. Br J Haematol 2008;140:36-45. [PubMed]
- Richardson SJ, Eve HE, Copplestone JA, et al. Activity of thalidomide and lenalidomide in mantle cell lymphoma. Acta Haematol 2010;123:21-9. [Crossref] [PubMed]
- Hagner PR, Chiu H, Ortiz M, et al. Activity of lenalidomide in mantle cell lymphoma can be explained by NK cell-mediated cytotoxicity. Br J Haematol 2017;179:399-409. [Crossref] [PubMed]
- Fecteau J-F, Corral LG, Ghia EM, et al. Lenalidomide inhibits the proliferation of CLL cells via a cereblon/p21WAF1/Cip1-dependent mechanism independent of functional p53. Blood 2014;124:1637-44. [Crossref] [PubMed]
- Krönke J, Udeshi ND, Narla A, et al. Lenalidomide Causes Selective Degradation of IKZF1 and IKZF3 in Multiple Myeloma Cells. Science 2014;343:301-5. [Crossref] [PubMed]
- Lu G, Middleton RE, Sun H, et al. The Myeloma Drug Lenalidomide Promotes the Cereblon-Dependent Destruction of Ikaros Proteins. Science 2014;343:305-9. [Crossref] [PubMed]
- Ramsay AG, Clear AJ, Kelly G, et al. Follicular lymphoma cells induce T-cell immunologic synapse dysfunction that can be repaired with lenalidomide: implications for the tumor microenvironment and immunotherapy. Blood 2009;114:4713-20. [Crossref] [PubMed]
- Ramsay AG, Clear AJ, Fatah R, et al. Multiple inhibitory ligands induce impaired T-cell immunologic synapse function in chronic lymphocytic leukemia that can be blocked with lenalidomide: establishing a reversible immune evasion mechanism in human cancer. Blood 2012;120:1412-21. [Crossref] [PubMed]
- Song K, Herzog BH, Sheng M, et al. Lenalidomide inhibits lymphangiogenesis in preclinical models of mantle cell lymphoma. Cancer Res 2013;73:7254-64. [Crossref] [PubMed]
- Qian Z, Zhang L, Cai Z, et al. Lenalidomide synergizes with dexamethasone to induce growth arrest and apoptosis of mantle cell lymphoma cells in vitro and in vivo. Leuk Res 2011;35:380-6. [Crossref] [PubMed]
- Lenz G, Dreyling M, Hoster E, et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG). J Clin Oncol 2005;23:1984-92. [Crossref] [PubMed]
- Forstpointner R, Unterhalt M, Dreyling M, et al. Maintenance therapy with rituximab leads to a significant prolongation of response duration after salvage therapy with a combination of rituximab, fludarabine, cyclophosphamide, and mitoxantrone (R-FCM) in patients with recurring and refractory follicular and mantle cell lymphomas: Results of a prospective randomized study of the German Low Grade Lymphoma Study Group (GLSG). Blood 2006;108:4003-8. [Crossref] [PubMed]
- Kluin-Nelemans HC, Hoster E, Hermine O, et al. Treatment of older patients with mantle-cell lymphoma. N Engl J Med 2012;367:520-31. [Crossref] [PubMed]
- Abrahamsson A, Albertsson-Lindblad A, Brown PN, et al. Real world data on primary treatment for mantle cell lymphoma: a Nordic Lymphoma Group observational study. Blood 2014;124:1288-95. [Crossref] [PubMed]
- Boross P, Leusen JH. Mechanisms of action of CD20 antibodies. Am J Cancer Res 2012;2:676-90. [PubMed]
- Hernandez-Ilizaliturri FJ, Reddy N, Holkova B, et al. Immunomodulatory Drug CC-5013 or CC-4047 and Rituximab Enhance Antitumor Activity in a Severe Combined Immunodeficient Mouse Lymphoma Model. Clin Cancer Res 2005;11:5984-92. [Crossref] [PubMed]
- Wu L, Adams M, Carter T, et al. lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clin Cancer Res 2008;14:4650-7. [Crossref] [PubMed]
- Zhang L, Qian Z, Cai Z, et al. Synergistic antitumor effects of lenalidomide and rituximab on mantle cell lymphoma in vitro and in vivo. Am J Hematol 2009;84:553-9. [Crossref] [PubMed]
- Habermann TM, Lossos IS, Justice G, et al. Lenalidomide oral monotherapy produces a high response rate in patients with relapsed or refractory mantle cell lymphoma. Br J Haematol 2009;145:344-9. [Crossref] [PubMed]
- Witzig TE, Luigi Zinzani P, Habermann TM, et al. Long-term analysis of phase II studies of single-agent lenalidomide in relapsed/refractory mantle cell lymphoma. American Journal of Hematology 2017;92:E575-83. [Crossref] [PubMed]
- Eve HE, Carey S, Richardson SJ, et al. Single-agent lenalidomide in relapsed/refractory mantle cell lymphoma: results from a UK phase II study suggest activity and possible gender differences. Br J Haematol 2012;159:154-63. [Crossref] [PubMed]
- Zinzani PL, Vose JM, Czuczman MS, et al. Long-term follow-up of lenalidomide in relapsed/refractory mantle cell lymphoma: subset analysis of the NHL-003 study. Ann Oncol 2013;24:2892-7. [Crossref] [PubMed]
- Goy A, Kalayoglu Besisik S, Drach J, et al. Longer-term follow-up and outcome by tumour cell proliferation rate (Ki-67) in patients with relapsed/refractory mantle cell lymphoma treated with lenalidomide on MCL-001(EMERGE) pivotal trial. Br J Haematol 2015;170:496-503. [Crossref] [PubMed]
- Goy A, Sinha R, Williams ME, et al. Single-agent lenalidomide in patients with mantle-cell lymphoma who relapsed or progressed after or were refractory to bortezomib: phase II MCL-001 (EMERGE) study. J Clin Oncol 2013;31:3688-95. [Crossref] [PubMed]
- Arcaini L, Lamy T, Walewski J, et al. Prospective subgroup analyses of the randomized MCL-002 (SPRINT) study: lenalidomide versus investigator's choice in relapsed or refractory mantle cell lymphoma. Br J Haematol 2018;180:224-35. [Crossref] [PubMed]
- Zaja F, De Luca S, Vitolo U, et al. Salvage treatment with lenalidomide and dexamethasone in relapsed/refractory mantle cell lymphoma: clinical results and effects on microenvironment and neo-angiogenic biomarkers. Haematologica 2012;97:416-22. [Crossref] [PubMed]
- Wang M, Fayad L, Wagner-Bartak N, et al. Lenalidomide in combination with rituximab for patients with relapsed or refractory mantle-cell lymphoma: a phase 1/2 clinical trial. Lancet Oncol 2012;13:716-23. [Crossref] [PubMed]
- Ruan J, Martin P, Shah B, et al. Lenalidomide plus Rituximab as Initial Treatment for Mantle-Cell Lymphoma. N Engl J Med 2015;373:1835-44. [Crossref] [PubMed]
- Ruan J, Martin P, Christos P, et al. Five-year follow-up of lenalidomide plus rituximab as initial treatment of mantle cell lymphoma. Blood 2018;132:2016-25. [Crossref] [PubMed]
- Houot R, Cartron G, Bijou F, et al. Obinutuzumab plus Lenalidomide (GALEN) for the treatment of relapse/refractory aggressive lymphoma: a phase II LYSA study. Leukemia 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Zaja F, Ferrero S, Stelitano C, et al. Second-line rituximab, lenalidomide, and bendamustine in mantle cell lymphoma: a phase II clinical trial of the Fondazione Italiana Linfomi. Haematologica 2017;102:e203-6. [Crossref] [PubMed]
- Albertsson-Lindblad A, Kolstad A, Laurell A, et al. Lenalidomide-bendamustine-rituximab in patients older than 65 years with untreated mantle cell lymphoma. Blood 2016;128:1814-20. [Crossref] [PubMed]
- Flinn IW, Mainwaring M, Peacock N, et al. Rituximab, Lenalidomide, and Bortezomib in the First-Line or Second-Line Treatment of Patients with Mantle Cell Lymphoma a Phase I/II Trial. Blood 2012;120:2748.
- Morrison VA, Jung SH, Johnson J, et al. Therapy with bortezomib plus lenalidomide for relapsed/refractory mantle cell lymphoma: Final results of a phase II trial (CALGB 50501). Leuk Lymphoma 2015;56:958-64. [Crossref] [PubMed]
- Jerkeman M, Eskelund CW, Hutchings M, et al. Ibrutinib, lenalidomide, and rituximab in relapsed or refractory mantle cell lymphoma (PHILEMON): a multicentre, open-label, single-arm, phase 2 trial. Lancet Haematol 2018;5:e109-16. [Crossref] [PubMed]
- Witzig TE, Wiernik PH, Moore T, et al. Lenalidomide oral monotherapy produces durable responses in relapsed or refractory indolent non-Hodgkin's Lymphoma. J Clin Oncol 2009;27:5404-9. [Crossref] [PubMed]
- U.S. Food and Drug Administration. Lenalidomide. U.S. Food and Drug Administration. Available online: https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm542791.htm. 2018. Accessed 5 June, 2013.
- Trněný M, Lamy T, Walewski J, et al. Lenalidomide versus investigator's choice in relapsed or refractory mantle cell lymphoma (MCL-002; SPRINT): a phase 2, randomised, multicentre trial. Lancet Oncol 2016;17:319-31. [Crossref] [PubMed]
- 10th ICML, Abstract 306, Lugano, Switzerland. Ann Oncol 2008;19:
- Chong EA, Ahmadi T, Aqui NA, et al. Combination of Lenalidomide and Rituximab Overcomes Rituximab Resistance in Patients with Indolent B-cell and Mantle Cell Lymphomas. Clin Cancer Res 2015;21:1835-42. [Crossref] [PubMed]
- Rummel MJ. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 2013;381:1203-10. [Crossref] [PubMed]
- Eskelund CW, Albertsson-Lindblad A, Kolstad A, et al. Lenalidomide plus bendamustine-rituximab does not overcome the adverse impact of TP53 mutations in mantle cell lymphoma. Haematologica 2018;103:e541-3. [Crossref] [PubMed]
- Nowakowski GS, LaPlant B, Macon WR, et al. Lenalidomide combined with R-CHOP overcomes negative prognostic impact of non-germinal center B-cell phenotype in newly diagnosed diffuse large B-Cell lymphoma: a phase II study. J Clin Oncol 2015;33:251-7. [Crossref] [PubMed]
- Ribrag V, Feugier P, Doorduijn J, et al. MCL-R2 elderly: a phase iii study of the European MCL NETWORK ASSESSING EFFICACY OF ALTERNATING IMMUNOCHEMOTHERAPY (R-CHOP / R-HAD) and a rituximab-lenalidomide maintenance. Hematological Oncology 2017;35:421. [Crossref]
- Robak T, Huang H, Jin J, et al. Bortezomib-based therapy for newly diagnosed mantle-cell lymphoma. N Engl J Med 2015;372:944-53. [Crossref] [PubMed]
- Raedler L. Velcade (Bortezomib) Receives 2 New FDA Indications: For Retreatment of Patients with Multiple Myeloma and for First-Line Treatment of Patients with Mantle-Cell Lymphoma. Am Health Drug Benefits 2015;8:135-40. [PubMed]
- Dimopoulos M, Wang M, Maisnar V, et al. Response and progression-free survival according to planned treatment duration in patients with relapsed multiple myeloma treated with carfilzomib, lenalidomide, and dexamethasone (KRd) versus lenalidomide and dexamethasone (Rd) in the phase III ASPIRE study. J Hematol Oncol 2018;11:49. [Crossref] [PubMed]
- Dreyling M, Campo E, Hermine O, et al. Newly diagnosed and relapsed mantle cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv62-71. [Crossref] [PubMed]
- Cheah CY, Nastoupil LJ. Lenalidomide, idelalisib, and rituximab are unacceptably toxic in patients with relapsed/refractory indolent lymphoma. Blood 2015;125:3357-9. [Crossref] [PubMed]
- Smith SM, Pitcher BN, Jung SH, et al. Safety and tolerability of idelalisib, lenalidomide, and rituximab in relapsed and refractory lymphoma: the Alliance for Clinical Trials in Oncology A051201 and A051202 phase 1 trials. Lancet Haematol 2017;4:e176-82. [Crossref] [PubMed]
- Davids MS, Roberts AW, Wierda WG, et al. Long-Term Follow-up of Patients with Mantle Cell Lymphoma Treated with Venetoclax Monotherapy. Blood 2018;132:2883.
- Hilal T, Wang Z, Almader-Douglas D, et al. Rituximab maintenance therapy for mantle cell lymphoma: A systematic review and meta-analysis. Am J Hematol 2018;93:1220-6. [Crossref] [PubMed]
- Zaja F, Ferrero S, Stelitano C, et al. Rituximab, lenalidomide, bendamustine second line therapy in mantle cell lymphoma: a phase ii study of the Fondazione Italiana Linfomi (FIL). 13th International Conference on Malignant Lymphoma; June 17; Palazzo Dei Congressi, Lugano, Schweiz: Hematological Oncology, 2015.
- Witzig TE, Nowakowski GS, Habermann TM, et al. A comprehensive review of lenalidomide therapy for B-cell non-Hodgkin lymphoma. Ann Oncol 2015;26:1667-77. [Crossref] [PubMed]
- Hitz F, Zucca E, Pabst T, et al. Rituximab, bendamustine and lenalidomide in patients with aggressive B-cell lymphoma not eligible for anthracycline-based therapy or intensive salvage chemotherapy - SAKK 38/08. Br J Haematol 2016;174:255-63. [Crossref] [PubMed]
- Carrier M, Le Gal G, Tay J, et al. Rates of venous thromboembolism in multiple myeloma patients undergoing immunomodulatory therapy with thalidomide or lenalidomide: a systematic review and meta-analysis. J Thromb Haemost 2011;9:653-63. [Crossref] [PubMed]
- Yamshon S, Christos PJ, Demetres M, et al. Venous thromboembolism in patients with B-cell non-Hodgkin lymphoma treated with lenalidomide: a systematic review and meta-analysis. Blood Adv 2018;2:1429-38. [Crossref] [PubMed]
- Dimopoulos MA, Richardson PG, Brandenburg N, et al. A review of second primary malignancy in patients with relapsed or refractory multiple myeloma treated with lenalidomide. Blood 2012;119:2764-7. [Crossref] [PubMed]
- Palumbo AP, Delforge M, Catalano J, et al. Incidence of second primary malignancy (SPM) in melphalan-prednisone-lenalidomide combination followed by lenalidomide maintenance (MPR-R) in newly diagnosed multiple myeloma patients (pts) age 65 or older. J Clin Oncol 2011;29:8007. [Crossref]
- Attal M, Lauwers-Cances V, Marit G, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med 2012;366:1782-91. [Crossref] [PubMed]
- Holstein SA, Jung SH, Richardson PG, et al. Updated analysis of CALGB (Alliance) 100104 assessing lenalidomide versus placebo maintenance after single autologous stem-cell transplantation for multiple myeloma: a randomised, double-blind, phase 3 trial. Lancet Haematol 2017;4:e431-42. [Crossref] [PubMed]
- Shah BK, Khanal A. Second Primary Malignancies in Mantle Cell Lymphoma: A US Population-based Study. Anticancer Res 2015;35:3437-40. [PubMed]
- Delfau-Larue MH, Klapper W, Berger F, et al. High-dose cytarabine does not overcome the adverse prognostic value of CDKN2A and TP53 deletions in mantle cell lymphoma. Blood 2015;126:604-11. [Crossref] [PubMed]
- Aukema SM, Hoster E, Rosenwald A, et al. Expression of TP53 is associated with the outcome of MCL independent of MIPI and Ki-67 in trials of the European MCL Network. Blood 2018;131:417-20. [Crossref] [PubMed]
- Eskelund CW, Dahl C, Hansen JW, et al. TP53 mutations identify younger mantle cell lymphoma patients who do not benefit from intensive chemoimmunotherapy. Blood 2017;130:1903-10. [Crossref] [PubMed]
- Fink AM, Bahlo J, Robrecht S, et al. Lenalidomide maintenance after first-line therapy for high-risk chronic lymphocytic leukaemia (CLLM1): final results from a randomised, double-blind, phase 3 study. Lancet Haematol 2017;4:e475-86. [Crossref] [PubMed]
- Tilly H, Morschhauser F, Salles G, et al. Phase 1b study of lenalidomide in combination with rituximab-CHOP (R2-CHOP) in patients with B-cell lymphoma. Leukemia 2013;27:252-5. [Crossref] [PubMed]
- Wang ML, Lee H, Chuang H, et al. Ibrutinib in combination with rituximab in relapsed or refractory mantle cell lymphoma: a single-centre, open-label, phase 2 trial. Lancet Oncol 2016;17:48-56. [Crossref] [PubMed]
- Mehta RS, Di Stasi A, Hosing C, et al. Lenalidomide-induced graft-vs.-Leukemia effect in a patient with chronic lymphocytic leukemia who relapsed after allogeneic stem cell transplant. Clin Lymphoma Myeloma Leuk 2014;14:e105-9. [Crossref] [PubMed]
- Martin P, Maddocks K, Leonard JP, et al. Postibrutinib outcomes in patients with mantle cell lymphoma. Blood 2016;127:1559-63. [Crossref] [PubMed]
Cite this article as: Albertsson-Lindblad A, Jerkeman M. Is there a role for immunomodulatory drugs in the treatment of mantle cell lymphoma? Ann Lymphoma 2019;3:2.


