
Front-line therapy in elderly patients with mantle cell lymphoma
Introduction
Mantle cell lymphoma (MCL) is a biologically and clinically heterogeneous subtype of B-cell non-Hodgkin lymphoma characterized by cyclin D1 overexpression resulting from the t(11;14)(q13;q32) or, in a small percentage of patients, by overexpression of alternative G1 cyclins, cyclin D2 or D3 (1,2). The clinical spectrum ranges from in situ and indolent subtypes to the more common advanced-stage and symptomatic variants, commonly featuring both nodal as well as extranodal involvement of marrow, gastrointestinal tract, other organs, and/or soft tissue sites. Initial therapy for patients with MCL has evolved considerably in recent years with an appreciable divergence in therapies that can be offered to elderly patients compared to their younger counterparts with otherwise-similar disease features. This review will examine recent clinical trial results that inform and improve upon current standards of care, and those that will impact management of elderly MCL patients in the coming years.
Chemoimmunotherapy regimens
Anthracycline-based therapy with rituximab, and the role of maintenance therapy
As with diffuse large B-cell lymphoma (DLBCL), CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) was identified early on as an active regimen in patients with MCL. Although not achieving the same durable responses seen with DLBCL, this regimen was tolerated reasonably well in elderly patients with MCL and became a standard of care in older patients able to receive combination anthracycline-based chemotherapy (3,4). With the advent of rituximab, subsequent trials combining the anti-CD20 monoclonal antibody with standard multi-agent regimens such as CHOP have shown notable improvement in outcomes without significant additive toxicity (5). Despite high overall complete and partial response rates, the duration of response was typically only 15–18 months.
Incorporating bortezomib in combination with R-CHOP (with vincristine omitted; the VR-CAP regimen) showed a significant advantage in outcomes vs. R-CHOP in a phase III trial; these results are reviewed below.
Borrowing from studies in the relapsed/refractory setting, fludarabine-based therapy was considered early on as a plausible alternative to R-CHOP and led to the randomized study comparing this regimen with the combination of fludarabine, cyclophosphamide, and rituximab (FCR) (6). This phase III multicenter study enrolled 560 patients across Europe, age >65 and with a new diagnosis of MCL, who were randomized between these two regimens. A second randomization followed for those achieving partial or complete response (PR or CR) to maintenance therapy with either rituximab once every 8 weeks until relapse or unacceptable toxicity versus interferon-alpha. Those receiving FCR induction ultimately had significantly shorter median overall survival (OS; 47% vs. 62% at 4 years) and higher rates of hematologic toxicity during therapy; infection rates were similar. Across all patients undergoing second randomization to receive maintenance therapy (n=316, 56% of initial enrollment), rituximab maintenance was associated with a significant 45% decrease in risk of death or disease progression. For responding patients receiving R-CHOP induction therapy, rituximab maintenance was associated with statistically improved OS (87% vs. 63%) compared to interferon (Table 1). With long-term follow-up these data were confirmed with a persisting prolongation of OS and PFS (7).
Table 1
| Trial name | Regimen | Comparison | Maintenance | Total pts | Median f/u (m) | ORR (%) | CR (%) | PFS | OS | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| MCL elderly | RCHOP | FCR | Rituximab vs.
|
560 | 37 | 86 vs. 78 | 49 vs. 53 | 28 vs. 26 m*; |
62 vs. 47% |
Kluin-Nelemans et al., NEJM 2012 |
| STiL | BR | RCHOP | None | 514 | 45 | 93 vs. 91 | 40 vs. 30 | 69.5 vs. 31.2 m (HR 0.58) | (median not reached) | Rummel et al., Lancet 2013 |
| BRIGHT | BR | RCHOP, RCVP | None | 447 | 65 | 97 vs. 91 | 31 vs. 25 | 65.5% vs. 55.8% |
81.7% vs. 85.0% (at 5 years) | Flinn et al., Blood 2014; |
| LYM-3002 | VR-CAP | R-CHOP | None | 487 | 82 | 92 vs. 89 | 53 vs. 42 | 24.7 vs. 14.4 m (HR 0.63) | 90.7 vs. 55.7 m (HR 0.66) | Robak et al., NEJM 2015; |
*, time to treatment failure, RCHOP
This study defined two critical points in the standard of care for elderly patients with MCL. First, it confirmed R-CHOP to be an effective and better-tolerated therapy versus fludarabine-based therapy. Second, it established a role for maintenance rituximab therapy in MCL as a viable means to improve disease-free and overall survival following combination chemoimmunotherapy with R-CHOP. This maintenance paradigm has since been used as a model for various other combination regimens, as described in later sections.
While R-CHOP followed by maintenance rituximab [now typically given for 2–3 years rather than indefinitely, as utilized in the above phase III trial (6)] remains an option for front-line therapy in elderly MCL patients without anthracycline contraindications, it largely has been supplanted by other regimens and emerging therapies, as detailed below.
Bendamustine-based therapy
Prior to the fall of the Berlin Wall in 1989, many chemotherapy regimens in East Germany utilized bendamustine, a derivative of nitrogen mustard developed there in the early 1960s. Given its generally good tolerability profile, bendamustine re-emerged in a number of combination regimens during the past 20 years, including bendamustine-rituximab (BR) for B-cell lymphomas. Two phase III studies have directly evaluated front-line BR for patients with MCL, each of which also included patients with various indolent lymphomas (Table 1).
The German STiL trial (8) compared BR with R-CHOP, enrolling 94 patients with MCL over age 65; younger MCL patients were referred to alternative clinical trials which incorporated autologous SCT consolidation. Patients received 6 cycles of combination chemoimmunotherapy, without maintenance rituximab, with improvement in progression-free survival of 35 vs. 22 months favoring BR over R-CHOP as well as significantly lower rates of both hematologic toxicities and infectious complications in the BR group.
The international BRIGHT study (9) compared BR to R-CHOP or R-CVP (cyclophosphamide, vincristine, prednisone) in patients with previously untreated indolent NHL or MCL. Subgroup analysis of patients with MCL showed higher CR rates in those receiving BR (50%, n=34) compared to 27% with R-CHOP (n=22) or R-CVP (n=11). However, while non-inferiority (primary outcome) was demonstrated for BR across the combined study population, it was notably not powered to make statistical inferences for the MCL subgroup. The results of the BRIGHT study with 5-year follow-up were recently reported, which verified an ongoing PFS benefit for MCL (HR 0.65 for BR vs. R-CHOP, and HR 0.80 vs. R-CVP), though notably there was no significant difference in OS (10), likely related to the availability of additional effective treatment options at relapse. Five MCL patients in each study arm received maintenance rituximab, too few to draw conclusions as to effect. No long-term monitoring or collection of adverse events was obtained during the follow-up period of the BRIGHT study, although across all indolent NHL and MCL patients enrolled (BR =224, R-CHOP/R-CVP =223) there were more secondary malignancies with BR, primarily squamous or basal cell skin cancers. There was also a trend for higher mortality, mostly infectious or cardiac, among BR-treated patients.
The allowance of the less-effective R-CVP as an option in the control arm for BRIGHT has been cited as an important qualifier of the BR results for MCL in that study (11). Nonetheless, both BRIGHT and STiL showed high rates of response and response duration as well as tolerability for BR among elderly patients with MCL. Based upon these results, this regimen is now established as a front-line treatment option in this population.
Serious or fatal infections with bendamustine have emerged during and after treatment completion as a significant concern, and are often delayed by 6–9 months or longer after initiating therapy or during anti-CD20 maintenance therapy. This is thought to be related especially to prolonged lymphodepletion of CD4+ T-cell subsets, although both B- and T-cell depletion are commonly noted when bendamustine is combined with anti-CD20 antibody therapies (12). Bacterial, viral, fungal, and other opportunistic infections such as Pneumocystis jirovecii pneumonia have all been reported in this context (13). A recent meta-analysis also demonstrated that infection rates may be higher than initially reported with bendamustine (14), and the follicular lymphoma GALLIUM trial showed increased infections and mortality with bendamustine versus CVP or CHOP chemotherapy combined with rituximab or obinutuzumab (15). Oncologists thus may consider incorporating antimicrobial prophylaxis into bendamustine-based treatment plans when treating elderly patients.
Today, bendamustine/rituximab is the most commonly used front-line regimen in elderly MCL patients, especially for those with contraindications to anthracycline-based therapy. Later sections will discuss various iterations and additions to this backbone, and most current front-line studies in this patient population will compare therapy to a BR-based standard-of-care arm.
Although maintenance rituximab following R-CHOP induction showed a significant benefit (6), improvement in PFS and OS with post-BR maintenance has not yet been confirmed in the limited trial data available. It’s use has been extrapolated from the R-CHOP study and from other regimens (including after autologous SCT) or in other B-cell malignancies (6,16-18). In the randomized MAINTAIN trial, preliminary results showed no benefit in PFS after 5-year follow-up for maintenance rituximab ×2 years (n=59) versus observation (n=61) following BR induction (19).
Cytarabine
In younger and medically fit patients, high-dose cytarabine has been shown to be a highly effective component of front-line therapy in MCL. The MCL Younger study (20), an international phase III trial in previously-untreated stage II-IV MCL patients below age 65 compared R-CHOP with alternating R-CHOP/R-DHAP (dexamethasone, high-dose cytarabine, cisplatin) prior to autologous SCT with cytarabine-based conditioning. Results of this study showed marked improvement in time to treatment failure (9.1 vs. 3.9 years), along with anticipated increase in treatment-related toxicities (hematologic, febrile neutropenia, renal) in those receiving cytarabine. Given its efficacy in MCL as well as its toxicity potential, various attempts have been made to modify cytarabine-based therapy for elderly patients.
Combining cytarabine with BR using reduced-intensity bendamustine (70 mg/m2), Visco et al. studied the so-called R-BAC regimen in 40 elderly patients (median age 70) with previously untreated MCL or having relapsed after one line of therapy (21). Following an initial dose-escalation stage with 6 patients to determine the optimal dose of cytarabine (800 mg/m2, later reduced to 500 mg/m2), 29 of 34 patients receiving R-BAC during phase II completed 4 or more cycles of therapy. Though reversible myelosuppression was frequent and 5 patients experienced grade 3/4 infectious complications, treatment was otherwise relatively well-tolerated and highly-effective. All previously-untreated patients achieved response, with a CR rate of 95%; ORR including relapsed refractory patients was 90%. With a median of 26 months follow-up at time of reporting, median PFS had not yet been reached and 2-year PFS in the previously-untreated cohort remained 100%. A subsequent multi-center study using this same regimen showed that, of 57 patients receiving R-BAC (median age 71), 52 achieved a CR after 4–6 cycles and 74% of patients were alive and disease-free at 35 months (22).
The impressive results of these studies show that careful application of cytarabine to existing combination regimens can be done safely and effectively, and R-BAC is currently being studied in ongoing trials. Similarly, a phase III study of alternating R-CHOP/R-cytarabine-dexamethasone (R-HAD) versus R-CHOP, followed by rituximab alone or rituximab plus lenalidomide maintenance, is currently underway through the European Mantle Cell Network (NCT 01865110).
Non-cytotoxic chemotherapy regimens and combinations of targeted agents plus chemoimmunotherapy
Lenalidomide
Borrowing from the relapsed/refractory setting (23,24), the immunomodulatory agent lenalidomide was tested in the front-line setting for elderly MCL patients in a small, multi-institution phase II study in combination with rituximab (25). Thirty-eight patients (median age 65, 71% male) received a starting dose of 20 mg lenalidomide daily, given 21 days on/7 days off for 12 cycles. Dosage was escalated to 25 mg daily after cycle 1 if well-tolerated and decreased to 15 mg daily after completion of induction. This was paired with rituximab 375 mg/m2 given weekly for the first 4 weeks of therapy, then every other 28-day cycle until disease progression.
After a median of 30 months follow-up, patients achieved an ORR of 92% with a 64% CR rate. Two-year PFS was 85% with 2-year overall survival remaining very high at 97%. Adverse events noted with this combination included rash (29%), cytopenias (grade 3–4: neutropenia in 50%, thrombocytopenia in 13%, anemia in 11%), and a “tumor flare” inflammatory syndrome in 11% which occurred early in therapy and resolved with supportive care measures. Quality-of-life assessment using the FACT-Lym were also assessed pre-treatment, post-induction, and during maintenance therapy. Though not statistically significant, survey outcomes at each point were stable or trended towards improvement across age, baseline performance status, MIPI score, and clinical response. Five-year follow-up of this trial found that 27/36 patients were able to complete at least 3 years of therapy, and that 8 of 10 evaluable patients were negative for minimal residual disease. PFS and OS at 3 years were 80% and 90%, respectively, with estimated 5-year rates of 64% and 77% (26).
This rituximab plus lenalidomide combination has three important implications for front-line therapy. First, it offers a “biologic only” front-line option which can be especially applicable for frail patients unable to receive conventional chemotherapy. This comes in contrast to rituximab monotherapy, which has relatively poor single-agent efficacy in MCL (27). Second, the synergistic efficacy of lenalidomide and rituximab shown in this study has reinforced the reported preclinical and clinical synergy achieved by adding lenalidomide to rituximab; this may extend to maintenance therapy with this combination following other induction regimens and thus provide additional clinical benefit (28,29). The latter is the focus of two key ongoing trials in Europe (NCT01865110) and in the US (ECOG 4411 trial, NCT01415752; see below), which are expected to report preliminary data in the coming year. However, the third implication cannot be ignored: regimens like this combination will cause marked increases in costs of treatment.
Bortezomib
Approved as single-agent therapy for MCL in the relapsed/refractory setting, the proteasome inhibitor bortezomib has been applied to front-line treatment of MCL in a number of different combinations. An early phase II study added bortezomib to 6 cycles of standard R-CHOP for patients with DLBCL or MCL, the latter group enrolling 36 patients with a median age of 66 (30). Though achieving an ORR of 81% and CR/unconfirmed CR rate of 64% in the intention-to-treat analysis, MCL patients experienced significant added toxicity with 5 of 36 relatively-healthy patients requiring major modifications to therapy for treatment-related complications.
The French phase II GOELAMS study used bortezomib in combination with rituximab, infusional doxorubicin, chlorambucil, and dexamethasone in elderly, previously-untreated MCL patients (31). Very similar to the previous study with bortezomib + R-CHOP, this RiPAD+C combination was considerably toxic for elderly patients, with nearly a third of patients requiring hospitalization for therapy-related complications and 7 of 39 experiencing grade 3 neuropathy. Despite achieving an ORR of 79% and CR rate of 59%, this regimen is rarely used in clinical practice.
Replacing the vincristine with bortezomib (1.3 mg/m2, 4 doses per cycle), Robak et al. compared the so-called VR-CAP combination to standard R-CHOP over 6–8 cycles in a phase III study which enrolled 487 patients with untreated MCL (32). No maintenance therapy was given with either arm. Median age was 66 years, and all patients were considered ineligible for high-dose chemotherapy and autologous SCT in order to enroll. After a median follow-up of 40 months, those receiving VR-CAP showed significant improvements in CR rate (53% vs. 42%) and progression-free survival [24.7 vs. 14.4 months; hazard ratio (HR) 0.63]. A recent update of this trial with a median follow-up of 82 months showed a significant increase in OS for VR-CAP of 91 vs. 56 months for R-CHOP (HR 0.66, P=0.001) (33). In terms of toxicity, there was an increase in hematologic toxicity with VR-CAP, especially grade 3–4 thrombocytopenia, but roughly equal rates of treatment-related neuropathy. No emergent serious adverse events were observed with addition of bortezomib to the modified R-CHOP backbone, although with longer follow-up there were two second primary malignancies in the VR-CAP arm (lung and gastric cancer) and one grade 2 pneumonia in the R-CHOP arm.
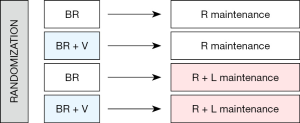
ECOG 1411 is a phase III trial assessing the role of two agents—bortezomib and lenalidomide, both active in MCL—at key positions in front-line therapy (see schema; Figure 1). Patients age 60 and older were randomized to BR with or without bortezomib induction, followed by rituximab with or without lenalidomide maintenance therapy for a total of 2 years. Study enrollment was completed and results are anticipated in 2020.
Bruton tyrosine kinase (BTK) inhibitors
With increased understanding of the B-cell receptor (BCR) pathway and its role in the oncogenesis and persistence of MCL, various signaling enzymes including BTK have emerged as potential targets in our evolving approach to this disease (34). Ibrutinib is an oral, irreversible inhibitor of BTK which binds at its phosphorylation site and has shown significant activity in the relapsed/refractory setting (35,36). Side effects notably include rash, arthralgias, atrial fibrillation, as well as bleeding complications which can be severe to life threatening (37). Initially prescribed as a single agent, combinations of ibrutinib with rituximab in both relapsed MCL (38) and other B-cell malignancies such as lymphoplasmacytic lymphoma (39) have shown synergistic efficacy without any significant emerging toxicities from the combination.
Multiple studies are planned or ongoing to assess the role of ibrutinib in the front-line setting, both alone (NCT03282396) and as part of multi-agent regimens (rituximab/lenalidomide/ibrutinib - NCT03232307; bendamustine/rituximab/ibrutinib - NCT01776840). The ongoing MCL Younger Trial (NCT02858258) is also testing ibrutinib in the front-line setting, albeit with a more intensive immunochemotherapy induction regimen than is applicable to elderly patients. The second generation BTK inhibitor acalabrutinib was also recently shown to have activity in the relapsed/refractory setting, and notably has far lower rates of bleeding complications and atrial fibrillation than the first-generation ibrutinib (40). Though neither of these agents are currently FDA-approved in the front-line setting, BTK inhibitors show significant promise and further study is warranted to assess their efficacy and tolerability as part of initial MCL-directed therapy, especially in elderly patient populations.
Venetoclax
The BCL-2 inhibitor venetoclax is another emerging therapy with potent clinical activity in MCL which is being studied in various combinations. Side effects are generally less frequent than with BTK and PI3K inhibitors, with the notable exception of tumor lysis syndrome which can be severe and warrants slow, step-wise dose-escalation when initiating venetoclax therapy. There is appreciable synergy with BTK inhibition in preclinical models (41), which has supported the combination of venetoclax with ibrutinib in the relapsed/refractory setting in published (42) and in an ongoing clinical trial (NCT03112174). As it is generally well-tolerated and highly active in MCL, venetoclax is a rational consideration for future combination studies in the front-line setting, especially for elderly patients.
Treatment approaches in the very frail elderly MCL patient
There is relatively little clinical trial data that specifically addresses this patient population, who necessarily require a careful approach to balance disease control and amelioration of MCL-related symptoms with treatment risk and toxicity. In the patient without overt lymphoma-related symptoms observation is preferred, while symptomatic patients may be offered single-agent rituximab or rituximab plus lenalidomide (see above) (26). Cytotoxic chemotherapy is typically avoided in the very frail patient population, but some experience is emerging with BTK inhibitors although caution and close monitoring is warranted due to the risk of serious adverse events such as atrial fibrillation or bleeding. Finally, involved field radiotherapy can be very helpful for local symptoms in select cases.
Conclusions
Elderly patients with MCL have several effective front-line therapeutic options, and multiple ongoing studies expected to report out in the near future may better define the optimal treatment approach in this population. Today, deciding the ideal front-line regimen should be based on extent of medical comorbidities, overall fitness to receive intensive induction chemotherapy, and for select, highly fit elderly patients, consideration of autologous SCT in first remission. R-CHOP with maintenance rituximab and BR are both considered standards of care, as is VR-CAP, although traditional R-CHOP is being increasingly supplanted by the latter regimens, or by lenalidomide plus rituximab in the frail elderly patient. Patient selection and consideration of medical comorbidities are essential when counseling patients as to therapeutic options, which should also include watchful waiting for the subset of patients with slow paced, lower burden and asymptomatic MCL.
The coming years will see continued evolution of front-line and maintenance therapy for elderly patients with MCL, including a broader incorporation of novel agents at various points in treatment as ongoing trial results are reported. It is further anticipated that biomarkers such as p53 mutation status and minimal residual disease analyses will be incorporated into clinical algorithms of risk-adapted therapy.
Acknowledgments
Funding: This work was supported in part by the Lymphoma Research Fund, University of Virginia, Hematology/ Oncology Division.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Martin Dreyling) for the series “Future Directions for Mantle Cell Lymphoma” published in Annals of Lymphoma. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aol.2019.06.02). The series “Future Directions for Mantle Cell Lymphoma” was commissioned by the editorial office without any funding or sponsorship. ME Williams serves as an unpaid editorial board member of Annals of Lymphoma, and reports grants from Allos, grants and personal fees from Celgene, grants and personal fees from Gilead Sciences, grants and personal fees from Janssen, grants and personal fees from Pharmacyclics, grants and personal fees from TG Therapeutics, personal fees from Abbvie, personal fees from Astra-Zeneca, personal fees from Kite, personal fees from Juno, personal fees from Verastem, personal fees from Seattle Genetics, personal fees from Sandoz, personal fees from Xian Janssen, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127:2375-90. [Crossref] [PubMed]
- Martín-Garcia D, Navarro A, Valdés-Mas R, et al. CCND2 and CCND3 hijack immunoglobulin light-chain enhancers in cyclin D1- mantle cell lymphoma. Blood 2019;133:940-51. [Crossref] [PubMed]
- Nickenig C, Dreyling M, Hoster E, et al. Combined cyclophosphamide, vincristine, doxorubicin, and prednisone (CHOP) improves response rates but not survival and has lower hematologic toxicity compared with combined mitoxantrone, chlorambucil, and prednisone (MCP) in follicular and mantle cell lymphomas. Cancer 2006;107:1014-22. [Crossref] [PubMed]
- Dreyling M, Hiddemann W, European MCL. Network. Current treatment standards and emerging strategies in mantle cell lymphoma. Hematology Am Soc Hematol Educ Program 2009;542-51. [Crossref] [PubMed]
- Coiffier B, Lepage E, Brière J, et al. CHOP Chemotherapy plus Rituximab Compared with CHOP Alone in Elderly Patients with Diffuse Large-B-Cell Lymphoma. N Engl J Med 2002;346:235-42. [Crossref] [PubMed]
- Kluin-Nelemans HC, Hoster E, Hermine O, et al. Treatment of Older Patients with Mantle-Cell Lymphoma. N Engl J Med 2012;367:520-31. [Crossref] [PubMed]
- Hoster E, Kluin-Nelemans H, Hermine O, et al. Rituximab Maintenance after First-Line Immunochemotherapy in Mantle Cell Lymphoma: Long-Term Follow-up of the Randomized European MCL Elderly Trial. Blood 2017;130:153.
- Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 2013;381:1203-10. [Crossref] [PubMed]
- Flinn IW, van der Jagt R, Kahl BS, et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood 2014;123:2944-52. [Crossref] [PubMed]
- Flinn IW, van der Jagt R, Kahl B, et al. First-Line Treatment of Patients With Indolent Non-Hodgkin Lymphoma or Mantle-Cell Lymphoma With Bendamustine Plus Rituximab Versus R-CHOP or R-CVP: Results of the BRIGHT 5-Year Follow-Up Study. J Clin Oncol 2019;37:984-91. [Crossref] [PubMed]
- Kluin-Nelemans JC, Doorduijn JK. What is the optimal initial management of the older MCL patient? Best Pract Res Clin Haematol. 2018;31:99-104. [Crossref] [PubMed]
- Gafter-Gvili A, Polliack A. Bendamustine associated immune suppression and infections during therapy of hematological malignancies. Leuk Lymphoma 2016;57:512-9. [Crossref] [PubMed]
- Gafter-Gvili A, Ribakovsky E, Mizrahi N, et al. Infections associated with bendamustine containing regimens in hematological patients: a retrospective multi-center study. Leuk Lymphoma 2016;57:63-9. [Crossref] [PubMed]
- Gafter-Gvili A, Gurion R, Raanani P, et al. Bendamustine-associated infections-systematic review and meta-analysis of randomized controlled trials. Hematol Oncol 2017;35:424-31. [Crossref] [PubMed]
- Marcus R, Davies A, Ando K, et al. Obinutuzumab for the First-Line Treatment of Follicular Lymphoma. N Engl J Med 2017;377:1331-44. [Crossref] [PubMed]
- Ahmadi T, McQuade J, Porter D, et al. Potential prolongation of PFS in mantle cell lymphoma after R-HyperCVAD: auto-SCT consolidation or rituximab maintenance. Bone Marrow Transplant 2012;47:1082-6. [Crossref] [PubMed]
- Le Gouill S, Thieblemont C, Oberic L, et al. Rituximab after Autologous Stem-Cell Transplantation in Mantle-Cell Lymphoma. N Engl J Med 2017;377:1250-60. [Crossref] [PubMed]
- Salles G, Seymour JF, Offner F, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet (London, England) 2011;377:42-51. [Crossref] [PubMed]
- Rummel MJ, Knauf W, Goerner M, et al. Two years rituximab maintenance vs. observation after first-line treatment with bendamustine plus rituximab (B-R) in patients with mantle cell lymphoma: First results of a prospective, randomized, multicenter phase II study (a subgroup study of the StiL NHL7-2008 MAINTAIN trial). J Clin Oncol 2016;34:7503. [Crossref]
- Hermine O, Hoster E, Walewski J, et al. Addition of high-dose cytarabine to immunochemotherapy before autologous stem-cell transplantation in patients aged 65 years or younger with mantle cell lymphoma (MCL Younger): a randomised, open-label, phase 3 trial of the European Mantle Cell Lymphoma Network. Lancet 2016;388:565-75. [Crossref] [PubMed]
- Visco C, Finotto S, Zambello R, et al. Combination of rituximab, bendamustine, and cytarabine for patients with mantle-cell non-Hodgkin lymphoma ineligible for intensive regimens or autologous transplantation. J Clin Oncol 2013;31:1442-9. [Crossref] [PubMed]
- Visco C, Chiappella A, Nassi L, et al. Rituximab, bendamustine, and low-dose cytarabine as induction therapy in elderly patients with mantle cell lymphoma: a multicentre, phase 2 trial from Fondazione Italiana Linfomi. Lancet Haematol 2017;4:e15-23. [Crossref] [PubMed]
- Eve HE, Carey S, Richardson SJ, et al. Single-agent lenalidomide in relapsed/refractory mantle cell lymphoma: Results from a UK phase II study suggest activity and possible gender differences. Br J Haematol 2012;159:154-63. [Crossref] [PubMed]
- Goy A, Sinha R, Williams ME, et al. Single-Agent Lenalidomide in Patients With Mantle-Cell Lymphoma Who Relapsed or Progressed After or Were Refractory to Bortezomib: Phase II MCL-001 (EMERGE) Study. J Clin Oncol 2013;31:3688-95. [Crossref] [PubMed]
- Ruan J, Martin P, Shah B, et al. Lenalidomide plus Rituximab as Initial Treatment for Mantle-Cell Lymphoma. N Engl J Med 2015;373:1835-44. [Crossref] [PubMed]
- Ruan J, Martin P, Christos P, et al. Five-year follow-up of lenalidomide plus rituximab as initial treatment of mantle cell lymphoma. Blood 2018;132:2016-25. [Crossref] [PubMed]
- Ghielmini M, Schmitz S-FH, Cogliatti S, et al. Effect of single-agent rituximab given at the standard schedule or as prolonged treatment in patients with mantle cell lymphoma: a study of the Swiss Group for Clinical Cancer Research (SAKK). J Clin Oncol 2005;23:705-11. [Crossref] [PubMed]
- Wang M, Fayad L, Wagner-Bartak N, et al. Lenalidomide in combination with rituximab for patients with relapsed or refractory mantle-cell lymphoma: a phase 1/2 clinical trial. Lancet Oncol 2012;13:716-23. [Crossref] [PubMed]
- Kritharis A, Coyle M, Sharma J, et al. Lenalidomide in non-Hodgkin lymphoma: biological perspectives and therapeutic opportunities. Blood 2015;125:2471-6. [Crossref] [PubMed]
- Ruan J, Martin P, Furman RR, et al. Bortezomib Plus CHOP-Rituximab for Previously Untreated Diffuse Large B-Cell Lymphoma and Mantle Cell Lymphoma. J Clin Oncol 2011;29:690-7. [Crossref] [PubMed]
- Houot R, Le Gouill S, Ojeda Uribe M, et al. Combination of rituximab, bortezomib, doxorubicin, dexamethasone and chlorambucil (RiPAD+C) as first-line therapy for elderly mantle cell lymphoma patients: results of a phase II trial from the GOELAMS. Ann Oncol 2012;23:1555-61. [Crossref] [PubMed]
- Robak T, Huang H, Jin J, et al. Bortezomib-Based Therapy for Newly Diagnosed Mantle-Cell Lymphoma. N Engl J Med 2015;372:944-53. [Crossref] [PubMed]
- Robak T, Jin J, Pylypenko H, et al. Frontline bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone (VR-CAP) versus rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in transplantation-ineligible patients with newly diagnosed mantle cell lymphoma: final overall survival results of a randomised, open-label, phase 3 study. Lancet Oncol 2018;19:1449-58. [Crossref] [PubMed]
- Arora PC, Portell CA. Novel therapies for relapsed/refractory mantle cell lymphoma. Best Pract Res Clin Haematol 2018;31:105-13. [Crossref] [PubMed]
- Cinar M, Hamedani FS, Mo Z, et al. Bruton tyrosine kinase is commonly overexpressed in mantle cell lymphoma and its attenuation by Ibrutinib induces apoptosis. Leuk Res 2013;37:1271-7. [Crossref] [PubMed]
- Wang ML, Rule S, Martin P, et al. Targeting BTK with Ibrutinib in Relapsed or Refractory Mantle-Cell Lymphoma. N Engl J Med 2013;369:507-16. [Crossref] [PubMed]
- Mock J, Kunk PR, Palkimas S, et al. Risk of Major Bleeding with Ibrutinib. Clin Lymphoma Myeloma Leuk 2018;18:755-61. [Crossref] [PubMed]
- Jain P, Romaguera J, Srour SA, et al. Four-year follow-up of a single arm, phase II clinical trial of ibrutinib with rituximab (IR) in patients with relapsed/refractory mantle cell lymphoma (MCL). Br J Haematol 2018;182:404-11. [Crossref] [PubMed]
- Dimopoulos MA, Tedeschi A, Trotman J, et al. Phase 3 Trial of Ibrutinib plus Rituximab in Waldenström’s Macroglobulinemia. N Engl J Med 2018;378:2399-410. [Crossref] [PubMed]
- Wang M, Rule S, Zinzani PL, et al. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): A single-arm, multicentre, phase 2 trial. Lancet 2018;391:659-67. [Crossref] [PubMed]
- Axelrod M, Ou Z, Brett LK, et al. Combinatorial drug screening identifies synergistic co-targeting of Bruton’s tyrosine kinase and the proteasome in mantle cell lymphoma. Leukemia 2014;28:407-10. [Crossref] [PubMed]
- Tam CS, Anderson MA, Pott C, et al. Ibrutinib plus Venetoclax for the Treatment of Mantle-Cell Lymphoma. N Engl J Med 2018;378:1211-23. [Crossref] [PubMed]
Cite this article as: Orellana-Noia VM, Kluin-Nelemans JC, Williams ME. Front-line therapy in elderly patients with mantle cell lymphoma. Ann Lymphoma 2019;3:8.


