
Targeting CDK4/6 in mantle cell lymphoma
Introduction
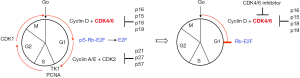
A hallmark of mantle cell lymphoma (MCL) is aberrant cyclin D1 expression caused by a t(11;14)(q13;q32) chromosomal translocation (1-4). One D-type cyclin (cyclin D1, D2 or D3) assembles with one cyclin-dependent kinase 4 (CDK4) or CDK6 to phosphorylate Rb in mammalian cells. This leads to the release of E2F transcription factors from phosphorylated Rb to orchestrate the expression of genes that promote the progression of the cell cycle from G1 to S phase, such as CCNA2 (cyclin A), TK1 (thymidine kinase) and proliferating cell nuclear antigen (PCNA), and a multitude of cellular functions (5). In turn, the cyclin D-CDK4/6 activity is kept in check by four physiologic CDK4/6 inhibitors (p16INK4a, p15INK4b, p18INK4c and p19INK4d) for programmed cell cycle progression (Figure 1). Alteration of the cyclin D-Rb pathway in MCL was first reported by Dreyling and colleagues, who discovered by fluorescence in situ hybridization (FISH) that CDKN2A (encoding p16INK4a) and RB1 (encoding Rb, the substrate of CDK4 and CDK6) were frequently deleted (41%) in primary MCL cells, and that deletion of CDKN2A, but not RB1, correlated with proliferation as determined by Ki67 expression (6). Gene expression profiling further suggested that differences in cyclin D1 mRNA abundance act in synergy with deletions at the INK4a/ARF locus to determine tumor proliferation rate and survival (7). Subsequently, Ki67 was shown to be a prognostic indicator for the patients treated with immunochemotherapy in MCL (8). Targeting the cell cycle, therefore, represents a rational approach to MCL therapy. Although cell cycle cancer therapy was ineffective due to a lack of selective and effective drugs in the past, this landscape has changed with the advent of selective and potent small-molecule oral CDK4/6 inhibitors. Here, we review the anti-tumor activities and clinical data of selective CDK4/6 inhibitors in MCL, and discuss the potential to harness this class of selective CDK4/6 inhibitors to advance precision medicine-based therapy and gain new insights into drug resistance in MCL and other cancers.
Targeting CDK4/6 in cancer
There are three oral small-molecule reversible CDK4/6 inhibitors that have been approved by the FDA for breast cancer treatment (Figure 2A). Palbociclib (PD 0332991, Ibrance), the first CDK4/6 selective inhibitor, was identified in a high throughput screen (9), and subsequently shown to be highly specific for cyclin D-CDK4 in a KinomeScan against 468 serine-threonine kinases including lipid kinases (Di Liberto M, Huang X, and Chen-Kiang S, 2019 unpublished data). Abemaciclib (LY2835219) appears less selective based on a KinomeScan on the same platform (10). The specificity of ribociclib (LEE011) (11) is not yet available (Figure 2A).
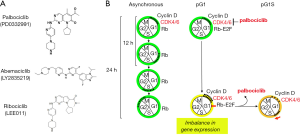
It was in primary human bone marrow myeloma cells that palbociclib was first demonstrated to inhibit CDK4/6 selectively and induce reversible early G1 cell cycle arrest in primary human cancer cells ex vivo (12). This complemented the findings that palbociclib suppressed tumor progression in vivo in xenografts of human MCL, multiple myeloma, acute myeloid leukemia (AML) and breast cancer cell lines in severe combined immunodeficiency (SCID) mice (12-15), and in immunocompetent mouse models of multiple myeloma (16) and T cell acute leukemia (17). Mechanistically, induction of prolonged early G1 arrest (pG1) by sustained inhibition of CDK4/6 with palbociclib beyond the time required for progression to S phase not only prevented cell cycle progression, but also restricted the expression of genes to those programmed for early G1 only (Figure 2B) (18). This caused an imbalance in gene expression that reprogrammed cancer cells for killing by diverse clinically relevant agents ex vivo and in animal models, including dexamethasone and bortezomib in myeloma (12,16,18), cytarabine in AML (19) and inhibitors of PI3K and BTK in MCL (20,21). Reinforcing the CDK4/6 specificity, expression of Rb (p105, substrate of CDK4 and CDK6) is indispensable for palbociclib’s activity in human cells, and this cannot be substituted by the related p107 or p130 (18). Collectively, these preclinical studies provide a compelling rationale for targeting CDK4/6 in cancer therapy and exploring the underpinnings.
Targeting CDK4/6 with palbociclib in MCL
In an unbiased whole transcriptome sequencing (WTS) analysis of MCL cells isolated from 73 patient samples and normal peripheral B cells (PBCs) from six healthy donors, CCND1 (encoding cyclin D1) mRNA was highly expressed in primary MCL cells, as expected from the t(11;14) chromosomal translocation, but not in PBCs (Figure 3) (Huang X, Di Liberto M and Chen-Kiang S, 2019 unpublished data). CDK4 mRNA was elevated in MCL cells compared with PBCs, whereas CDK6 mRNA was barely detectable in MCL cells even in those that were cycling based on MKI67 mRNA expression (Figure 3). This is in contrast to coordinated induction of CDK4 and CDK6 mRNAs in PBCs upon cell cycle entry following B cell receptor (BCR) engagement and CD40 stimulation (Figure 3). SOX11 and CD5 mRNAs were expressed at varying levels in MCL cells in most cases, but not in normal PBCs, while CD19 was expressed in MCL cells and normal PBCs alike, as expected of B lineage cells (Figure 3). As a consequence of aberrant cyclin D1 and preferential CDK4 expression, cyclin D1 mainly assembles with CDK4 to promote cell cycle progression in MCL cells, reminiscent of selective cyclin D1-CDK4 pairing in a subset of myeloma cases (22), and highlights the physiologic relevance of targeting CDK4 in MCL.

Consistent with this possibility, in the first disease-specific clinical trial of palbociclib, inhibition of CDK4/6 with palbociclib not only induced early G1 arrest initially in all patients with previously treated MCL but also elicited clinical responses with tumor regression in some patients (23). In this multicenter phase Ib study of 17 patients, the majority of patients (71%) were considered to have intermediate- or high-risk disease according to the MCL International Prognostic Index (MIPI) and were heavily pretreated with a median of 3 prior therapies, including 18% with prior autologous stem cell transplantation (ASCT). An objective response was observed in 3 patients (18%) treated with an oral daily dose of 125 mg for 21 days of a 28-day cycle, including one complete response (CR) and two partial responses (PR), in addition to 7 patients with stable disease (SD). While the median progression-free survival (PFS) was 4 months, responding patients experienced a duration of response (DOR) of 18 months or greater. Treatment was generally well tolerated with the most common adverse events being primarily hematologic, including neutropenia (41%) and thrombocytopenia (29%).
Patients in this trial underwent sequential biopsies prior to treatment and at week 3. Dual immunohistochemical staining (IHC) demonstrated that among cyclin D1-positive cells, treatment with palbociclib resulted in a significant decrease in Ki67 and CDK4/6-specific phospho-Rb (pS807/S811). Together with the reduction in uptake of thymidine seen by 18F-fluorothymidine positron emission tomography (FLT-PET) imaging, which corresponded more closely to clinical response than changes in uptake of glucose seen by 18F-fluorodeoxyglucose (FDG)-PET, these data are consistent with induction of early G1 arrest as a consequence of CDK4/6 inhibition in MCL patients.
Despite the modest clinical activity, this trial demonstrated through its well-designed correlative studies that CDK4/6 inhibition has a biological impact on MCL. Tumor regression in responding patients, particularly in one patient with a CR for more than 30 months, further suggests a mechanistic link between palbociclib-induced pG1 and cell death, as indicated in preclinical studies (18). As the first disease-specific clinical trial of a selective CDK4/6 inhibitor, it laid the groundwork for future studies of CDK4/6 in MCL as well as cancers of solid tissue origin, such as breast cancer. The reported trials of CDK inhibitors in MCL are summarized in Table 1 and discussed below.
Table 1
| Agent(s) | Design | No. patients | Patient characteristics | Results | AEs, most common and of interest |
|---|---|---|---|---|---|
| Palbociclib (23) | Phase Ib, multicenter | 17 | 24% high-risk MIPI, median of 3 prior therapies, 18% post-ASCT | ORR 18% (CR 6%) | Neutropenia (36% grade ≥3), thrombocytopenia (24% grade ≥3), fatigue (35%) |
| PFS 4 months | |||||
| Palbociclib + Bortezomib (24) | Phase I, single-center | 19 | 11% high-risk MIPI, median of 3 prior therapies | ORR 24% (CR 6%) | Neutropenia (63% grade ≥3), thrombocytopenia (53% grade ≥3), lymphopenia (32% grade ≥3) |
| SD 30% | |||||
| Palbociclib + Ibrutinib (25) | Phase I, multicenter | 27 | 26% high risk MIPI, 54% Ki67 ≥30%, 44% post-ASCT | ORR 67% (CR 37%) | Neutropenia (41% grade ≥3), thrombocytopenia (30% grade ≥3) |
| 2-year PFS 59% | |||||
| 2-year OS 61% | |||||
| Abemaciclib (26) | Phase II, multicenter | 22 | 91% with stage III or IV disease, 82% with ≥2 prior therapies | ORR 23% (CR 0%) | Neutropenia (32% grade ≥3), thrombocytopenia (32% grade ≥3), diarrhea (55%) |
| Ribociclib (27) | Phase I, multicenter | 7 MCL (132 total) | Not defined for MCL cohort | ORR 0% | Not defined for MCL cohort; electrocardiogram QT prolongation (11%) |
| SD 0% | |||||
| AT7519M (28) | Phase II, multicenter | 12 | 92% stage IV disease, median of 2 prior therapies | ORR 27% (PR 18%) | One each of grade 3 vomiting and dyspnea |
| mDOR 4.5 months | |||||
| Flavopiridol (bolus) (29) | Phase II, multicenter | 30 | 37% untreated, rest with ≤2 prior therapies | ORR 11% (CR 0%) | Neutropenia (38% grade ≥3), diarrhea (97%, 30% grade ≥3), nausea (47%) |
| mDOR 3.4 months | |||||
| Flavopiridol (continuous) (30) | Phase I, single-center | 10 | All received 1 prior chemotherapy regimen | ORR 0% | Diarrhea (100%, 10% grade ≥3), fatigue (70%), anorexia (50%), metallic taste (40%) |
| SD 30% | |||||
| Flavopiridol (bolus + continuous) (31) | Phase I, single-center | 7 MCL (46 total) | Not defined for MCL cohort | ORR 29% (PR 29%) | 1 MCL patient with grade 3 TLS |
CDK, cyclin-dependent kinase; AE, adverse event; MIPI, Mantle Cell Lymphoma International Prognostic Index; ASCT, autologous stem cell transplant; ORR, objective response rate; CR, complete response; PFS, progression-free survival; SD, stable disease; OS, overall survival; MCL, mantle cell lymphoma; PR, partial response; mDOR, median duration of response; TLS, tumor lysis syndrome.
Palbociclib + bortezomib
Based on preclinical data indicating that induction of pG1 by CDK4/6 inhibition could sensitize cancer cells to killing by various partner drugs (12,16,18), a series of combination trials were initiated. The treatment schema of a phase I dose escalation study of palbociclib in sequential combination with bortezomib in patients with previously treated MCL was designed to capitalize on bortezomib killing of MCL cells during palbociclib-induced pG1 as well as synchronous transition of G1-arrested cells into S phase following cessation of palbociclib (24). Bortezomib was administered at a reduced dose (1 mg/m2) during dose escalation of palbociclib with only 4 days of overlap (days 8–11), which potentially minimizes the combined toxicity. The trial established palbociclib 125 mg on days 1–12 and bortezomib 1 mg/m2 on days 8, 11, 15, and 18 of each 21-day cycle as the recommended dose for further evaluation. Higher doses of the combination (palbociclib at 125 mg + bortezomib at 1.3 mg/m2) were unsurprisingly associated with increased toxicity: prohibitive myelosuppression as grade 3–4 events included neutropenia (63%), thrombocytopenia (53%), lymphopenia (32%), and anemia (11%). Of the seven patients treated at the recommended dose level, 4 remained progression-free for greater than 12 months including 1 patient with a CR that has lasted for over 7 years while remaining on single-agent palbociclib.
This single center phase I study demonstrated that selective inhibition of CDK4/6 in sequential combination with reduced-dose bortezomib is biologically active and tolerable in previously treated MCL. Only one patient progressed while receiving treatment and this patient subsequently achieved a PR in response to the BTK (Bruton’s tyrosine kinase) inhibitor ibrutinib (25), suggesting that the mechanisms mediating clinical responses to palbociclib and ibrutinib are distinct. The maintenance of a durable CR by palbociclib alone after 6 cycles of palbociclib + bortezomib invokes palbociclib as a potential maintenance therapy after achieving a CR to palbociclib-based combination therapy. It also raises intriguing questions as to how inhibiting two essential cell cycle enzymes can be tolerated, especially for as long as 7 years and continuing in one patient, and whether this could be attributed to periodic resumption of the cell cycle and cellular function following cessation of palbociclib for a brief period in each cycle of treatment. Most importantly, what are the genomic basis and mechanisms that discriminate sensitivity from resistance to targeting CDK4/6?
In the genomic era, longitudinal analysis of sequential patient samples before, during and after treatment is attainable. Studying MCL has an added advantage in rapid isolation of fresh tumor cells based on cell surface markers (Figure 3) for genomic and biochemical analysis, and for functional studies ex vivo. Such a longitudinal integrated analysis of WTS and whole exome sequencing (WES) in concert with functional analysis was undertaken in a single-agent ibrutinib therapy, which led to the discovery of a relapse-specific C418S BTK mutation in MCL (21). Analogous longitudinal functional genomics of MCL cells isolated from sequential lymph node biopsies of responders and non-responders prior to treatment, early on palbociclib treatment before administration of bortezomib, at the end of the first treatment cycle and on progression (of one patient) is ongoing. The results should shed light on genomic drivers that discriminate sensitivity from resistance to targeting CDK4/6 in combination therapy.
Palbociclib + ibrutinib
Ibrutinib is a standard of care for MCL (32). However, nearly half of all patients experience treatment failure during the first year, and this is often associated with a more aggressive disease (33). In addition to uncovering a relapse-specific C481S BTK mutation in MCL, longitudinal functional genomics of sequential MCL cells from individual patients further revealed activation of the PI3K pathway as a mechanism for ibrutinib resistance in patients with a wild-type BTK (21). Induction of pG1 by CDK4/6 inhibition can overcome ibrutinib resistance in primary human MCL cells and MCL cell lines expressing wild-type BTK (Figure 4) (21), in part by inactivating PI3K through activation of PIK3IP1, a negative regulator of PI3Kp110, and NF-κB in palbociclib-induced pG1 (20,21).
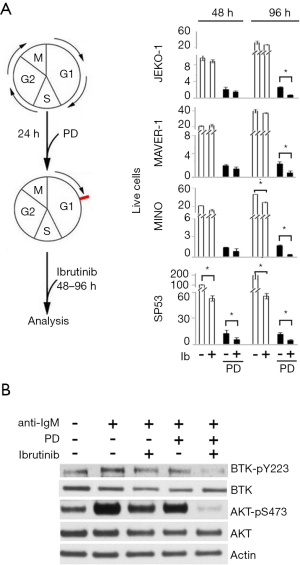
On this basis, a hypothesis-driven multicenter clinical trial was undertaken to test if inhibition of CDK4/6 can deepen and prolong the clinical response to ibrutinib while assessing the tolerability. This phase I study of palbociclib and ibrutinib combination therapy (PALIBR) in 27 patients with previously treated MCL demonstrated a CR rate of 37% and median PFS of 25.6 months (25), which appear better than might be expected based on studies of single-agent ibrutinib, despite a comparable objective response rate (ORR) of 67% (34). Among seven patients with a Ki67 higher than 30%, 5 responded including three patients with a CR. Among seven patients with a high MIPI, 4 responded including 1 with a CR (24). The maximum tolerated doses were ibrutinib 560 mg daily and palbociclib 100 mg on days 1–21 of a 28-day cycle. The combination had an acceptable safety profile, with a dose-limiting toxicity of grade 3 rash that led to discontinuation in two patients taking 125 mg of palbociclib. The most common grade 3 or 4 toxicities were hematologic, including neutropenia (41%) and thrombocytopenia (30%).
The observed activity was consistent with the hypothesis that induction of pG1 by CDK4/6 could deepen and prolong the clinical response to ibrutinib, including MCL patients with a high Ki67 and MIPI. A multicenter, phase II study is ongoing (NCT03478514) to further characterize the efficacy of this combination regimen. Longitudinal functional genomics to identify genomic drivers for resistance to both palbociclib and ibrutinib and for progression after a durable clinical response is underway.
Other CDK inhibitors
Abemaciclib
Abemaciclib is a broad-spectrum oral CDK4/6 inhibitor currently being studied in a single-arm phase II trial for patients with previously treated MCL. Preliminary results in 22 patients treated with oral abemaciclib 200 mg every 12 hours showed that five patients achieved PR (23%) and nine patients had SD (26). The most common adverse events (AEs) were diarrhea (55%), nausea (23%), vomiting (32%), and hematologic, including thrombocytopenia (55%), neutropenia (36%), and anemia (18%). Despite comparable clinical activity, these adverse effects differ from those of palbociclib considerably, most likely due to off-target effects caused by inhibition of serine-threonine kinases in addition to CDK4 and CDK6. How these features of abemaciclib might contribute to the clinical outcome, tolerability and durability either as a single agent or in combination therapy remain to be determined.
Ribociclib
Ribociclib is the third oral CDK4/6 inhibitor approved by the FDA in combination with an aromatase inhibitor for treatment of metastatic breast cancer. A total of seven patients with MCL were enrolled in a large phase I trial involving patients with a variety of previously treated solid tumors and lymphomas. The recommended dose for expansion was established at 600 mg daily with a 3-week-on/1-week-off schedule due to a lower rate of QT prolongation which occurs in a concentration-dependent manner. As expected, myelosuppression was the primary dose-limiting toxicity. There were no responses among the patients with MCL (27).
AT7519M
The Canadian Cancer Trials Group reported the results of a phase II study of AT7519M, a potent inhibitor of CDKs 1, 2, 4, 5, and 9, in patients with previously treated MCL (28). Dosed at 27 mg/m2/day twice weekly on weeks 1 and 2 (days 1, 4, 8, and 11) repeated every 3 weeks, the ORR of the 12 MCL patients was 27% with two patients achieving PR (18%) with a DOR of 4.5 months. Six patients (55%) had SD, including one patient who subsequently met PR criteria 9 months after discontinuation of AT7519M with no other therapy. Only two grade 3 AEs were reported: vomiting and dyspnea. Hematologic toxicity was mostly grade 1 and 2 neutropenia, anemia, and thrombocytopenia.
Voruciclib
Voruciclib, a potent inhibitor of CDK9 in addition to CDK4, CDK6 and CDK1 (35), is being studied in a phase Ib trial in patients with previously treated B-cell malignancies including an MCL cohort (ClinicalTrials.gov: NCT03547115). CDK9 is not a classical cell cycle regulator. It is a transcriptional factor that regulates the expression of MCL1 (a survival factor of the BCL2 family) as well as c-Myc, among other targets. As a broad-spectrum CDK inhibitor, voruciclib may block cell proliferation by inhibiting CDK4, CDK6 and CDK1, and CDK9 to impair cell survival by down-regulating MCL1. The therapeutic window of voruciclib will depend on how inhibiting multiple CDKs can be translated into a clinical response, and the tolerability and durability of this broad-spectrum CDK inhibitor.
Flavopiridol
One of the earliest clinical trials investigating CDK inhibitor therapy in MCL used flavopiridol, a broad CDK inhibitor, dosed at 50 mg/m2/day by intravenous bolus for 3 consecutive days every 21 days in a phase II trial of 30 patients with untreated or relapsed MCL (29). Three patients achieved a PR (11%) with a median DOR of 3.4 months, and 20 patients had SD (71%). Non-hematologic toxicities of all grades included diarrhea (97%), fatigue (73%), nausea (47%), and vomiting (27%). In addition, 11 patients (38%) experienced at least one grade 3 or 4 neutropenia. Due to potential issues with limited bioavailability following bolus dosing, alternative strategies using either a 72-hour continuous infusion schedule (30) or a hybrid dosing schedule of bolus followed by continuous infusion (31) were evaluated, both of which yielded similar safety profiles and similarly modest clinical activity. This collective experience is frequently cited as evidence against therapies that target CDKs, but should probably be more appropriately considered as evidence against a challenging drug and too broad a target.
Perspectives
The development of selective CDK4/6 inhibitors has changed the landscape in targeting the cell cycle in human cancer, most notably in breast cancer. Palbociclib, the first-in-class selective CDK4/6 inhibitor, remains the most specific of all CDK4/6 inhibitors to date. As such, it provides an unprecedented opportunity to target the cell cycle in mechanism-based clinical trials. Data emerging from three clinical trials of palbociclib are consistent with the hypothesis that induction of pG1 by CDK4/6 inhibition not only prevents proliferation of MCL cells, but also reprograms them for a deeper and more durable clinical response to the partner drug including patients with high Ki67 and high MIPI. Durable CR was observed in one patient treated with palbociclib alone for 30 months (23); in one patient for over 7 years while on treatment with palbociclib as a single agent after 6 cycles of palbociclib in sequential combination with reduced-dose bortezomib (24), and in ten patients (37%) in the palbociclib + ibrutinib clinical trial. Among them, 3 went on to transplant, 3 progressed after 3, 23 and 28 months, respectively, and 4 remained in CR for 2.5 to 5 years while on therapy (25). Although preliminary, these findings reinforce the potential of targeting CDK4/6, as well as the critical importance of determining the tumor intrinsic and extrinsic mechanisms that discriminate sensitivity from resistance to targeting CDK4/6 in MCL.
Integrative longitudinal analysis of WTS and WES data of individual patients before and during treatment, and after progression, has identified a relapse-specific BTK mutation in ibrutinib therapy (21). Analogous longitudinal functional genomics represents the best approach to discover driver genomic aberrations in addition to loss of RB1 in resistance to CDK4/6 inhibition and define the role of clonal evolution on progression following a CR or PR. It could also illuminate genes and signaling pathways that are programmed in G1 arrest to maintain a durable clinical response. Understanding the genomic basis for resistance is also a requisite for optimizing the selection and sequencing of a partner drug(s) with a CDK4/6 inhibitor in combination therapy.
Inhibition of CDK4/6 is not restricted to disease or cell lineage, suggesting that tumor-immune interactions are likely to be dynamically regulated by CDK4/6 inhibition and contribute to the clinical response. In line with this possibility, Goel and colleagues showed in cell lines, murine models, and patient-derived xenografts that treatment with either palbociclib or abemaciclib promoted cytotoxic T-cell mediated clearance of tumor cells (36). Deng and colleagues identified CDK4/6 inhibitors through a small-molecule screen as a class of compounds that could activate PD-1-overexpressing Jurkat T cells, and treatment with CDK4/6 inhibitors increased infiltration of CD4+ and CD8+ T cells as well as the levels of Th1 cytokines in a mouse model of lung cancer (37). Similarly, treatment with abemaciclib resulted in enhanced intratumoral T cell activation and inflammation in colorectal cancer cells (38). CDK4/6 inhibition was further shown to repress a tumor resistance program associated with T cell exclusion and immune evasion as determined by single-cell RNA sequencing in 33 melanoma tumors (39). Collectively, these studies illustrate the tumor-extrinsic mechanisms by which CDK4/6 inhibitors may enhance anti-tumor immunity. It will be important to see if these promising preclinical data translate into better efficacy in patients, particularly for groups who have had disappointing results to immune checkpoint inhibition alone, including MCL patients.
Longitudinal integrative analysis of single-cell RNA-seq and WTS and WES of purified MCL cells from the phase II palbociclib-ibrutinib clinical trial presents an ideal strategy to investigate tumor-immune interactions in the context of a clinical response and may shed light on the therapeutic potential of dual CDK4/6 and immune checkpoint inhibition in MCL.
Acknowledgments
We thank Kevin Wang for valuable inputs and critical reading of this manuscript, and Ariana Malave for administrative support.
Funding: This study was supported in part by a Conquer Cancer Foundation of ASCO/Frist Family Endowed Young Investigator Award in honor of Howard A. Burris, III, MD (C Lee), Translational Research Grants from V-Foundation (S Chen-Kiang, M Di Liberto, P Martin), NIH/NCI RO1CA18894 (S Chen-Kiang), MCL-RI Award (MCL7001-18) from The Leukemia & Lymphoma Society to S Chen-Kiang. Funding for this project has been provided by the Sarah Cannon Fund at the HCA Foundation (S Chen-Kiang, X Huang, M Di Liberto, P Martin), and NIH/NCI P01 CA21427401 (S Chen-Kiang, X Huang, M Di Liberto, P Martin).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Martin Dreyling) for the series “Future Directions for Mantle Cell Lymphoma” published in Annals of Lymphoma. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aol.2019.12.01). The series “Future Directions for Mantle Cell Lymphoma” was commissioned by the editorial office without any funding or sponsorship. Christina Lee reports grants from Conquer Cancer Foundation of ASCO, during the conduct of the study; personal fees from Targeted Healthcare Communications, outside the submitted work. Peter Martin reports personal fees from Bayer, personal fees from Beigene, personal fees from Celgene, personal fees from Cellectar, personal fees from AstraZeneca, personal fees from Janssen, personal fees from Karyopharm, personal fees from Kite, personal fees from Morphosys, personal fees from Regeneron, personal fees from Teneobio, personal fees from Verastem, outside the submitted work. Selina Chen-Kiang reports grants from National Cancer Institute, grants from Leukemia and Lymphoma Society, during the conduct of the study. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bosch F, Jares P, Campo E, et al. PRAD-1/cyclin D1 gene overexpression in chronic lymphoproliferative disorders: a highly specific marker of mantle cell lymphoma. Blood 1994;84:2726-32. [Crossref] [PubMed]
- de Boer CJ, van Krieken JH, Kluin-Nelemans HC, et al. Cyclin D1 messenger RNA overexpression as a marker for mantle cell lymphoma. Oncogene 1995;10:1833-40. [PubMed]
- Ott MM, Helbing A, Ott G, et al. bcl-1 rearrangement and cyclin D1 protein expression in mantle cell lymphoma. J Pathol 1996;179:238-42. [Crossref] [PubMed]
- Rosenberg CL, Wong E, Petty EM, et al. PRAD1, a candidate BCL1 oncogene: mapping and expression in centrocytic lymphoma. Proc Natl Acad Sci U S A 1991;88:9638-42. [Crossref] [PubMed]
- Kent LN, Leone G. The broken cycle: E2F dysfunction in cancer. Nat Rev Cancer 2019;19:326-38. [Crossref] [PubMed]
- Dreyling MH, Bullinger L, Ott G, et al. Alterations of the cyclin D1/p16-pRB pathway in mantle cell lymphoma. Cancer Res 1997;57:4608-14. [PubMed]
- Rosenwald A, Wright G, Wiestner A, et al. The proliferation gene expression signature is a quantitative integrator of oncogenic events that predicts survival in mantle cell lymphoma. Cancer Cell 2003;3:185-97. [Crossref] [PubMed]
- Determann O, Hoster E, Ott G, et al. Ki-67 predicts outcome in advanced-stage mantle cell lymphoma patients treated with anti-CD20 immunochemotherapy: results from randomized trials of the European MCL Network and the German Low Grade Lymphoma Study Group. Blood 2008;111:2385-7. [Crossref] [PubMed]
- Fry DW, Harvey PJ, Keller PR, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther 2004;3:1427-38. [PubMed]
- Gelbert LM, Cai S, Lin X, et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest New Drugs 2014;32:825-37. [Crossref] [PubMed]
- Rader J, Russell MR, Hart LS, et al. Dual CDK4/CDK6 inhibition induces cell-cycle arrest and senescence in neuroblastoma. Clin Cancer Res 2013;19:6173-82. [Crossref] [PubMed]
- Baughn LB, Di Liberto M, Wu K, et al. A novel orally active small molecule potently induces G1 arrest in primary myeloma cells and prevents tumor growth by specific inhibition of cyclin-dependent kinase 4/6. Cancer Res 2006;66:7661-7. [Crossref] [PubMed]
- Marzec M, Kasprzycka M, Lai R, et al. Mantle cell lymphoma cells express predominantly cyclin D1a isoform and are highly sensitive to selective inhibition of CDK4 kinase activity. Blood 2006;108:1744-50. [Crossref] [PubMed]
- Wang L, Wang J, Blaser BW, et al. Pharmacologic inhibition of CDK4/6: mechanistic evidence for selective activity or acquired resistance in acute myeloid leukemia. Blood 2007;110:2075-83. [Crossref] [PubMed]
- Finn RS, Dering J, Conklin D, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res 2009;11:R77. [Crossref] [PubMed]
- Menu E, Garcia J, Huang X, et al. A novel therapeutic combination using PD 0332991 and bortezomib: study in the 5T33MM myeloma model. Cancer Res 2008;68:5519-23. [Crossref] [PubMed]
- Sawai CM, Freund J, Oh P, et al. Therapeutic targeting of the cyclin D3:CDK4/6 complex in T cell leukemia. Cancer Cell 2012;22:452-65. [Crossref] [PubMed]
- Huang X, Di Liberto M, Jayabalan D, et al. Prolonged early G(1) arrest by selective CDK4/CDK6 inhibition sensitizes myeloma cells to cytotoxic killing through cell cycle-coupled loss of IRF4. Blood 2012;120:1095-106. [Crossref] [PubMed]
- Yang C, Boyson CA, Di Liberto M, et al. CDK4/6 Inhibitor PD 0332991 Sensitizes Acute Myeloid Leukemia to Cytarabine-Mediated Cytotoxicity. Cancer Res 2015;75:1838-45. [Crossref] [PubMed]
- Chiron D, Martin P, Di Liberto M, et al. Induction of prolonged early G1 arrest by CDK4/CDK6 inhibition reprograms lymphoma cells for durable PI3Kdelta inhibition through PIK3IP1. Cell Cycle 2013;12:1892-900. [Crossref] [PubMed]
- Chiron D, Di Liberto M, Martin P, et al. Cell-cycle reprogramming for PI3K inhibition overrides a relapse-specific C481S BTK mutation revealed by longitudinal functional genomics in mantle cell lymphoma. Cancer Discov 2014;4:1022-35. [Crossref] [PubMed]
- Ely S, Di Liberto M, Niesvizky R, et al. Mutually exclusive cyclin-dependent kinase 4/cyclin D1 and cyclin-dependent kinase 6/cyclin D2 pairing inactivates retinoblastoma protein and promotes cell cycle dysregulation in multiple myeloma. Cancer Res 2005;65:11345-53. [Crossref] [PubMed]
- Leonard JP, LaCasce AS, Smith MR, et al. Selective CDK4/6 inhibition with tumor responses by PD0332991 in patients with mantle cell lymphoma. Blood 2012;119:4597-607. [Crossref] [PubMed]
- Martin P, Ruan J, Furman R, et al. A phase I trial of palbociclib plus bortezomib in previously treated mantle cell lymphoma. Leuk Lymphoma 2019;60:2917-21. [Crossref] [PubMed]
- Martin P, Bartlett NL, Blum KA, et al. A phase 1 trial of ibrutinib plus palbociclib in previously treated mantle cell lymphoma. Blood 2019;133:1201-4. [Crossref] [PubMed]
- Morschhauser F, Bouabdallah K, Stilgenbauer S, et al. Clinical Activity of Abemaciclib (LY2835219), a Cell Cycle Inhibitor Selective for CDK4 and CDK6, in Patients with Relapsed or Refractory Mantle Cell Lymphoma. Blood 2014;124:3067. [Crossref]
- Infante JR, Cassier PA, Gerecitano JF, et al. A Phase I Study of the Cyclin-Dependent Kinase 4/6 Inhibitor Ribociclib (LEE011) in Patients with Advanced Solid Tumors and Lymphomas. Clin Cancer Res 2016;22:5696-705. [Crossref] [PubMed]
- Seftel MD, Kuruvilla J, Kouroukis T, et al. The CDK inhibitor AT7519M in patients with relapsed or refractory chronic lymphocytic leukemia (CLL) and mantle cell lymphoma. A Phase II study of the Canadian Cancer Trials Group. Leuk Lymphoma 2017;58:1358-65. [Crossref] [PubMed]
- Kouroukis CT, Belch A, Crump M, et al. Flavopiridol in Untreated or Relapsed Mantle-Cell Lymphoma: Results of a Phase II Study of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2003;21:1740-5. [Crossref] [PubMed]
- Lin TS, Howard OM, Neuberg DS, et al. Seventy-two hour continuous infusion flavopiridol in relapsed and refractory mantle cell lymphoma. Leuk Lymphoma 2002;43:793-7. [Crossref] [PubMed]
- Jones JA, Rupert AS, Poi M, et al. Flavopiridol can be safely administered using a pharmacologically derived schedule and demonstrates activity in relapsed and refractory non-Hodgkin's lymphoma. Am J Hematol 2014;89:19-24. [Crossref] [PubMed]
- Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med 2013;369:507-16. [Crossref] [PubMed]
- Martin P, Maddocks K, Leonard JP, et al. Postibrutinib outcomes in patients with mantle cell lymphoma. Blood 2016;127:1559-63. [Crossref] [PubMed]
- Rule S, Dreyling M, Goy A, et al. Outcomes in 370 patients with mantle cell lymphoma treated with ibrutinib: a pooled analysis from three open-label studies. Br J Haematol 2017;179:430-8. [Crossref] [PubMed]
- Dey J, Deckwerth TL, Kerwin WS, et al. Voruciclib, a clinical stage oral CDK9 inhibitor, represses MCL-1 and sensitizes high-risk Diffuse Large B-cell Lymphoma to BCL2 inhibition. Sci Rep 2017;7:18007. [Crossref] [PubMed]
- Goel S, DeCristo MJ, Watt AC, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature 2017;548:471-5. [Crossref] [PubMed]
- Deng J, Wang ES, Jenkins RW, et al. CDK4/6 Inhibition Augments Antitumor Immunity by Enhancing T-cell Activation. Cancer Discov 2018;8:216-33. [Crossref] [PubMed]
- Schaer DA, Beckmann RP, Dempsey JA, et al. The CDK4/6 Inhibitor Abemaciclib Induces a T Cell Inflamed Tumor Microenvironment and Enhances the Efficacy of PD-L1 Checkpoint Blockade. Cell Rep 2018;22:2978-94. [Crossref] [PubMed]
- Jerby-Arnon L, Shah P, Cuoco MS, et al. A Cancer Cell Program Promotes T Cell Exclusion and Resistance to Checkpoint Blockade. Cell 2018;175:984-997.e24. [Crossref] [PubMed]
Cite this article as: Lee C, Huang X, Di Liberto M, Martin P, Chen-Kiang S. Targeting CDK4/6 in mantle cell lymphoma. Ann Lymphoma 2020;4:1.


