
The complex pathology and differential diagnosis of splenic and nodal marginal zone lymphoma
Splenic marginal zone lymphoma (SMZL)
The term SMZL was coined to describe cases of small B-cell lymphoma involving the spleen with a perifollicular (marginal zone) pattern (1). Further molecular and cytogenetic studies have contributed to the recognition of this lymphoma type and a more precise definition of the disorder (2-10). SMZL appears to account for around 1–2% of all lymphomas. The median age at diagnosis is around 65 years, without gender predominance. Almost all patients have splenomegaly with some degree of bone marrow and peripheral blood involvement. Serum paraproteinemia (usually a low level of IgM) is observed in 10–28% of cases.
The diagnostic criteria for splenic marginal zone are clearly established by the WHO and are set out in the review of the Splenic International Study Group (2,11). Although findings in spleen, bone marrow and peripheral blood have been described, a precise diagnosis may require the careful integration of clinical findings with the morphological, immunophenotypic and molecular features of bone marrow and peripheral blood involvement, since diagnostic splenectomies are rarely performed nowadays.
Spleen
Splenic involvement is characterized by a lymphoid infiltrate with a micronodular pattern centered in the white pulp, with a variable degree of red pulp infiltration, involving cords and sinuses (Figure 1). Tumor nodules of the white pulp are centered on pre-existing follicles, leading to follicular replacement of germinal center cells by neoplastic cells. The tumoral cytology of these nodules is biphasic, and composed of an inner core of small lymphocytes, replacing the mantle and germinal center, surrounded by a rim of cells with moderately abundant pale cytoplasm, which is referred to as marginal zone differentiation. Marginal isolated large cells can be present in this area, which is where the proliferation takes place. In the red pulp, the majority of cells are small lymphocytes with round nuclei and scarce cytoplasm. Plasma cells with light-chain restriction may be observed within the germinal center and/or red pulp in some cases.
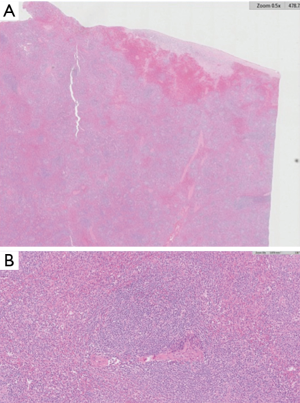
In a few cases, a higher frequency of large B-cells can be found within the marginal zone, the area where proliferation takes place in these tumors (12). Splenic hilar lymph node frequently shows lymphoma infiltration with a micronodular pattern, similar to splenic tissue, but with less conspicuous marginal zone differentiation (13).
Bone marrow
Bone marrow aspirate usually shows a mild degree of lymphoid infiltration. To establish a precise diagnosis of SMZL, a bone marrow biopsy is frequently required, because the aspirate is not always sufficient for an accurate diagnosis (2).
Bone marrow biopsy involvement is usually present, although sometimes it is only visible after CD20 staining. The main finding in bone marrow biopsy is the presence of a combination of intertrabecular nodules with intrasinusoidal infiltration and a pattern that mimics that observed in the spleen. Thus, the neoplastic nodules may show remnants of replaced germinal centers with CD21/CD23-positive follicular dendritic cells (FDCs) and aggregates of T-cells. Neoplastic cells in the bone marrow show some monocytoid features, with clear cytoplasm, but lack obvious marginal zone differentiation (14,15). Although an intrasinusoidal pattern can be observed in other B-cell lymphomas, including follicular lymphoma, mantle cell lymphoma, chronic lymphocytic leukemia, hairy cell leukemia, and very specially splenic red pulp lymphoma, the combination of intertrabecular nodules centered in the germinal center with intrasinusoidal infiltration is characteristic of SMZL (16).
Peripheral blood
Peripheral blood involvement shows small lymphocytes, some of which have small cytoplasmic projections, described as villous cells. Lymphoplasmacytoid cells and cells with a monocytoid appearance may be admixed in variable proportions. Villous cells are frequently observed in SMZL, but it is important to note that they can also be seen in other small cell lymphoproliferative diseases, such as FL and MCL.
In a pattern analogous to the differences observed between MBL and CLL, a subset of SMZL patients presents with monoclonal lymphocytosis with morphology, phenotype and molecular data consistent with SMZL, although only a proportion of them subsequently develop clinically active disease with splenomegaly (17). Currently, it is uncertain whether all SMZL cases have a previous indolent phase of MBL with an SMZL phenotype.
Lymph node
Peripheral lymph node involvement may also be the first clinical manifestation of the disease, although it is not frequent. The lymph node infiltration has a micronodular pattern with a cytological composition similar to splenic involvement, but without obvious marginal zone differentiation (13). Although morphological characteristics are similar in both lymph node localizations, there is greater architectural effacement in the peripheral lymph nodes, while the sinusoidal network of splenic hilar lymph nodes tends to be preserved (13).
Phenotype and flow cytometry immunophenotype
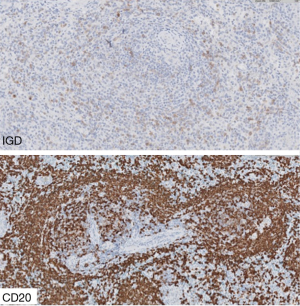
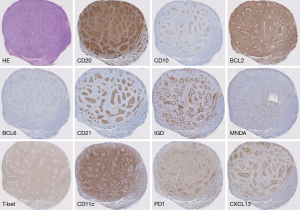
Immunohistochemical techniques show that the phenotype of the tumoral cells is positive for B-cell markers (CD20, PAX5, and others), very frequently IgM+ IgD+, with negativity for germinal center markers (CD10, BCL6), and lack of expression of MCL (CyclinD1), CLL (LEF1) and HCL (AnnexinA1) markers (Figures 2-4).
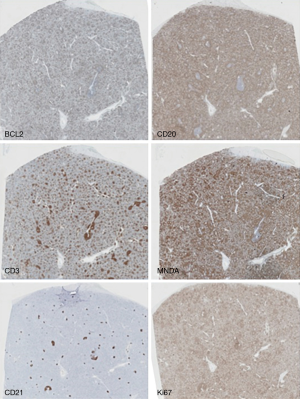
CD5 is expressed by a subset of SMZL cases in 25% of SMZLs (10,18). CD5-positive cases show a higher lymphocyte count at diagnosis and more frequent diffuse bone marrow infiltration (18), but prognosis and other features are similar in CD5-positive and CD5-negative cases.
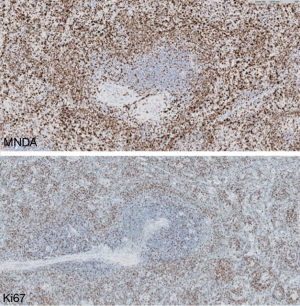
Staining with Ki67 shows a distinctive annular pattern, indicating the greater proliferation in the germinal center and marginal zone compartment (Figure 2).
Bcl2 staining is very useful for signaling the replacement of bcl2-negative, bcl6-positive germinal centers with bcl2-positive, bcl6-negative tumor cells.
FDC staining with antibodies for CD23 or CD21, highlights the preexisting follicles in the tumoral nodules, which are a useful diagnostic tool in bone marrow biopsy.
P53 is usually negative, although a few cases (10–25%) may exhibit the increased p53 expression that is commonly associated with p53 mutations.
Diagnosis of SMZL is facilitated by the demonstration of MNDA expression, a marginal zone marker (6), which is particularly useful for differentiating it from FL (Figure 4). In contrast to NMZL, IRTA1 is not found in SMZL (19).
There is a considerable amount of FCM data concerning SMZL. Most SMZL cases have Matutes CLL scores ranging from 0 to 2, a phenotype not seen in CLL. In the majority of patients, the immunophenotype is CD20+, CD22+, CD24+, CD27+, FMC7+, IgD+, IgM+, CD79b+, and some of them are DBA44+ (75%), CD11c+ (50%), CD23+ (30%), CD103+ (<10%), CD25+ (25%), and CD5+ (20%) (2).
IG stereotypes
The study of B-cell receptor immunoglobulin gene repertoires in MZ lymphomas supports the possible role of antigen selection in the pathogenesis of specific types of marginal zone B-cell lymphomas (20). Somatic mutations of IgVH genes have been observed in about half of SMZL cases (3,21,22). Several studies have revealed that one-third of SMZL cases make selective use of the IGVH1-2 segment (3,21,22), and Ig gene stereotypes are present in up to 30% of SMZLs (20,22).
IGHVH1-69 is also frequently found in SMZL and NMZL associated with HCV (23), confirming the role of infectious agents in shaping the B-cell repertoire. In this respect, tropical splenomegaly associated with malaria raises very interesting questions. The similarities between this disorder and SMZL have already been pointed out (24,25), and clinical experience suggests that SMZL cases in malaria patients may respond to antimalarial treatment.
Genetics and molecular findings
The most frequent cytogenetic alteration is deletion of the 7q22-36 (30–40%) chromosomal region, which occurs at a much higher frequency than observed in other B-cell neoplasms (8). It is a useful cytogenetic marker for this neoplasm, which may be used in conjunction with other morphological, phenotypic, and clinical features (8). Other known chromosomal alterations are gains of 3/3q, 1q, 6q, 8p, 9q, 13q, 21p, +12, and +18, losses of 6q, 14q, 1p, 8p, 13q, and 17p, and translocations involving 14q32, 8q24 (10,26). TNFAIP3 (A20) genomic loss has been reported in 8% of SMZLs (27).
Gene profiling studies have identified an SMZL signature, including upregulation of genes involved in apoptosis regulation, BCR and TNF signaling, and NFKB activation, such as SYK, BTK, BIRC3, TRAF3, TRAF5, CD40, and LTB. Genes associated with the splenic microenvironment, like SELL and LPXN, are also overexpressed. The TCL1, ARHH, AP-1, and NOTCH2 genes are also upregulated (9,28).
Whole-exome sequencing in SMZL reveals mutations in genes involved in marginal zone differentiation, NOTCH2, KLF2, and others (7,29,30). The most frequently mutated gene is KLF2, a transcription factor important for B-cell differentiation and NF-kB activation, is found in 20–42% of SMZL cases (31,32). All SMZL genetic studies coincide in showing a high frequency of mutations in NF-κB pathway genes: specifically, TNFAIP3, MYD88, TRAF3, CARD11, IKBKB, and BIRC3 (33). MYD88 L265P mutation can be found in otherwise typical SMZL cases (34,35), although SMZL cases with MYD88 mutation should be investigated for the presence of serum paraprotein and other factors suggesting LPL (36).
SMZL differential diagnosis
The diagnosis of SMZL requires the integration of clinical, morphological, phenotypic and molecular data. It is currently based on the examination of peripheral blood and bone marrow. Our diagnostic tools for recognizing SMZL have significantly diversified in recent years thanks to the identification of multiple IHC and molecular markers, but we still have some difficulties in making differential diagnoses with splenic red pulp diffuse small B-cell lymphoma (SRPL), lymphoplasmacytic lymphoma, and follicular lymphoma.
Spleen and bone marrow in SMZL both exhibit a micronodular pattern that recapitulates the phenomenon of marginal zone differentiation around reactive follicles, where germinal center remnants such as FDCs and T-cells can be found. The most useful differential markers are summarized in Table 1.
Table 1
| Marker | SMZL | SRPL | CLL | MCL | FL | HCL | LPL | NMZL |
|---|---|---|---|---|---|---|---|---|
| CD20 | >99% | >99% | >99%* | >99% | >99% | >99% | >99% | >99% |
| BCL6 | − | − | − | − | >90% | − | − | − |
| CD10 | − | − | − | − | 50–70% | − | 10–20% | − |
| MNDA (6,37-39) | 69–100% | ND | 13–65% | 78–82% | 5–20% | 0–67% | 25–83% | 54–75% |
| T-BET (40,41) | 33–75%* | ND | 90%* | 80%* | <1% | 100% | 33%* | 83% |
| CD11C (42-44) | 36–75% | 97% | − | − | − | 75–100% | − | 23% |
| IRTA1 (19,39,40) | 0–25% | ND | − | − | 0–7% | − | − | 43–73% |
| CD5 | 11–35% | 14–25% | >90% | >90% | <1% | <1% | <10% | 17% |
| LEF1 | − | − | >90% | <5% | <10% | − | − | − |
| Cyclin D1 | −** | −** | −** | >95% | <1% | >70% | −** | −** |
| Cyclin D3 (45) | <10% | 72% | − | <5% | ND | 14% | <10% | <10% |
| Annexin A1 | − | − | − | − | − | >90% | − | ND |
| CD103 (43,44,46) | − | − | − | − | − | >95% | − | − |
| t12 | 12% | − | 15–19% | 25% | 4–23% | 15% | 4% | 10–20% |
| 13q loss | − | − | 48–65% | 22–55% | <10% | − | 5–13% | <10% |
| 7q loss (8,10,47,48) | 39% | 7–14% | − | − | − | − | − | − |
| t14;18 | − | − | − | − | >80% | − | − | − |
| MYD88 (35,49) | 15% | − | <5% | − | − | − | 67–100% | <5% |
| IGVH1.2 (3,4,8,10) | 35% | 3–23% | − | − | − | − | − | − |
| NOTCH2 (29,30,50-53) | 20–25% | <10% | − | − | − | − | ND | 20% |
| KLF2 (31,51,53) | 12–25% | − | − | − | − | 16% | ND | 12–17% |
| BRAF-V600E | − | − | − | − | − | >97% | − | 16% |
| KMT2D (MLL2) | 8–11% | − | − | 14% | 89% | − | − | 34% |
| PTPRD (51) | − | ND | − | − | − | ND | − | 20% |
*, weak; **, scattered cells may be positive; −, negative; rare, exceptional cases may be positive. ND, not data/only isolated cases reported.
SRPL
Frequently preceded by an indolent phase of monoclonal lymphocytosis, this lymphoma presents clinically with splenomegaly, bone marrow and peripheral blood involvement, mimicking SMZL. In contrast to SMZL, however, splenectomy specimens of this type of lymphoma show diffuse involvement of the red pulp, with infiltration of cords and sinusoids and effacement of the white pulp, although some reactive follicles are occasionally present. The tumoral cytology is characterized by a monomorphous population of small cells, whereas SMZL exhibits a biphasic cytology. A pure sinusoidal infiltration pattern of bone marrow is also characteristic of SRPL.
Cyclin D3, CD180 and DBA44 are more frequently expressed in SRPL than in SMZL (45,50).
A variety of genetic and molecular data can help achieve an accurate diagnosis when results of splenectomy studies are not available. The 7q deletion and trisomies of chromosomes 3 and 18 are rare in SRPL. Sequencing studies have shown increased expression and recurrent CCND3 mutations in SRPL, which never occur in SMZL, and an absence of mutations of SMZL genes, like NOTCH2 and KLF2 (45,52,54). Aggressive SRPL lymphoma cases have been shown to carry NOTCH1, TP53, and MAP2K1 mutations (52).
There is evidence that SRPL is a markedly different disorder from SMZL; in contrast, it overlaps considerably with some cases classified as variant HCL.
HCLv diagnosis is based on the recognition in peripheral blood of cell features that are hybrids of prolymphocytic leukemia and classic HCL. Splenic infiltration has been described as a diffuse pattern, while the bone marrow pattern is basically intrasinusoidal (55). The BRAF V600 mutation is absent from HCLv, but activating mutations in the MAP2K1 gene have been described in 50% of these cases (56).
Lymphoplasmacytic lymphoma
LPL diagnosis is based on the integration of three features: bone marrow infiltration by small B-cells with plasmacytic differentiation, monoclonal paraproteinemia, and MYD88 L265P change.
The features of some cases with SMZL histology may partially overlap with these. For example, the MYD88 mutation has been found in a small proportion of SMZL cases, some of which usually exhibit slight monoclonal paraproteinemia (35,57).
The bone marrow histology of LPL is usually quite different from that found in SMZL; thus, LPL is favored in cases with an increased frequency of mast cells and an absence of conspicuous intrasinusoidal involvement (58). In addition, the combination of CD22 and CD25 by flow cytometry may be useful for differential diagnosis (59).
Follicular lymphoma
The differential diagnosis between SZML and follicular lymphoma does not usually pose problems due to the different clinical, phenotypic and molecular characteristics of the two types of neoplasm. However, there is a group of follicular lymphomas whose initial clinical presentation includes splenomegaly and involvement of bone marrow, but not of the peripheral lymph node, in which a diagnosis of SMZL may be suspected. In the splenectomy specimen, the nodules in FL are usually of variable size in contrast to the micronodular or miliary pattern in SMZL. The cytology composition and phenotype are also different, with centrocytes and centroblasts that express germinal center markers (CD10, BCL6) in FL compared with biphasic cytology and MNDA expression in SMZL. FISH for t(14;18) and 7q may also be useful in this context (60).
It is also important to mention the small group of cases of Bcl2-negative FL, in which NOTCH1 mutations have been associated with splenic involvement (61).
Bone marrow biopsies of FL usually show a characteristic paratrabecular pattern, which contrasts with the intertrabecular nodules of SMZL cases.
Other differential diagnoses are the following:
Marginal zone lymphoma, extranodal type
Exceptionally, marginal zone lymphoma, MALT-type, infiltrates the spleen. In these cases, the infiltration pattern is also micronodular, with rims of neoplastic cells occupying the marginal zone and surrounding preserved follicles.
In addition to the clinical features, cytogenetic alterations, such as the typical translocations present in MALT lymphomas, like t(14;18)(q21;q21), and the absence of IgD expression favor a diagnosis of MZL-MALT lymphoma.
Reactive conditions
The borderline between monoclonal B-lymphocytosis and MZL is arbitrary and reflects its direct adoption from CLL and MBL studies. However, for the time being, MBL is recognized because of the presence of a monoclonal B-cell count <5×109/L in the peripheral blood, in asymptomatic subjects without lymphadenopathy, organomegaly, other extramedullary involvement, or any other feature of a B-cell lymphoproliferative disorder (11). Diagnostic criteria for MBL do not change with the immunophenotype (62).
Monoclonal B-lymphocytosis is a common finding in HCV-positive patients and in the vast majority of the cases has not been shown to progress to SMZL (63).
Splenectomy specimens of persistent polyclonal B lymphocytosis (PPBL) have similar morphological features to those of SMZL (64,65), comprising a micronodular pattern and striking marginal differentiation. By definition, PPBL is a polyclonal and polytypic disorder.
Progression to large B-cell lymphoma and prognostic markers
Diffuse large B-cell lymphoma transformation has been observed in about 13% of SMZLs. It is most frequently localized in the peripheral lymph node, and neoplastic cells show a high level of bcl6 expression and high proliferative index (66). Isolated cases may progress to large B-cell lymphoma associated with EBV expression (67).
Several clinical scores have been proposed for diagnosing SMZL, including hemoglobin and platelet counts, a high lactate dehydrogenase level and the presence of extrahilar lymphadenopathy. Three risk groups with significantly different five-year lymphoma-specific survival (94%, 78%, and 69%, respectively) were identified (68,69).
Prognostic histological factors have not been identified or validated, but p53 alterations have been linked to clinical progression in four studies, which used distinct techniques (10,53,70,71). Shorter treatment-free survival also proved to be associated with NOTCH2 mutations in two studies (53,72). An ongoing massive high-throughput study has so far revealed that a cluster of SMZL cases, defined by NOTCH2 and/or KLF2 mutations and enriched in TNFAIP3 mutations and IGHV1-2*04 gene usage, behave more aggressively (ASH 2019).
Nodal marginal zone lymphoma (NMZL)
NMZL is a B-cell lymphoma originating from the lymph nodes that occupies the perifollicular and marginal zone and expands. It replaces the germinal centers, thereby mimicking the lymph nodes involved in marginal zone lymphoma of the extranodal or splenic types, although there is no evidence of extranodal or splenic disease (11). Its diagnosis requires the exclusion of splenic and MALT marginal zone lymphomas.
NMZL accounts for 1% of all non-Hodgkin lymphomas and 10% of marginal zone lymphomas. It is the least common marginal zone lymphoma subtype.
Morphology
MZL involves peripheral lymph nodes but can also involve the bone marrow and, occasionally, peripheral blood.
Lymph node
The morphology of NMZL is heterogeneous in the lymph node. The lymph nodes show lymphoid proliferation, with a growth pattern around pre-existing reactive follicles, with follicular colonization and with expansion into the interfollicular area (Figure 5). The morphological pattern of growth varies depending on the predominance of any of these components (peripheral, interfollicular or follicular colonization). In some cases, the growth pattern is diffuse, with effacement of the lymph node architecture, and in these cases, staining with dendritic markers is necessary to demonstrate residual germinal centers. A variant that resembles the progressively transformed centers, called the floral variant, has also been described (73).
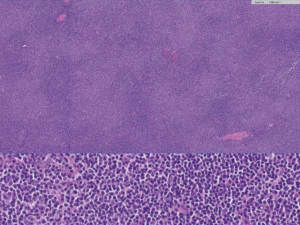
The tumoral cell composition is polymorphous, including small lymphocytes, marginal cells, monocytoid-type cells (medium-sized cells with a monocytoid nuclear shape and clear cytoplasm), plasma cells, plasmacytoid cells, and large cells in variable proportions. In some cases, more than 20% of the cells are large, requiring a differential diagnosis with large B-cell lymphoma (74). This is an issue that deserves special attention, because there is no agreement about the number of large cells necessary to justify the diagnosis of progression to large B-cell lymphoma (75). As some authors have pointed out, it seems appropriate to adopt the same criteria as applied to other small cell lymphomas, which consist of the presence of sheets of large cells (76).
Campo et al. recognized two patterns, one similar to that of lymph node infiltrated by SMZL, with loss of the mantle zone and IgD expression, and a second that is similar to the pattern in lymph nodes infiltrated by MALT lymphomas, in which the mantle is preserved and IgD is not expressed (77).
Bone marrow
Bone marrow involvement in NMZL has been reported in about 54% of cases (15). It usually shows an interstitial or nodular pattern, with an intertrabecular or paratrabecular localization. Intrasinusoidal infiltration, as seen in SMZL, has been reported (15). In contrast to SMZL, reactive germinal centers are not found in bone marrow biopsies, and the intrasinusoidal involvement is less frequent (78).
Immunophenotype
The majority of marginal zone lymphoma cells are positive for B-cell markers, negative for GC markers (CD10, BCL6), and positive for marginal zone markers such as MNDA. IRTA1, T-Bet, and CD11c (6,40). T-bet expression has been associated more frequently with monocytoid morphology (41). CD43 is also expressed in 20–45% cases. CD23 and CD5 are expressed rarely, but such cases lack LEF1 and CyclinD1 expression.
Staining with k67 highlights the perifollicular growth pattern (target or annular pattern) and staining with bcl2 helps to demonstrate the follicular colonization.
Expansion of PD1-positive cells have been reported in MZL cases, in a peculiar pattern that may lead to a suspicion of peripheral T-cell lymphoma (79) (Figure 6).
Genetic and molecular data
The precise molecular pathogenesis of NMZL remains poorly defined. Analyses of the mutational pattern of Ig heavy-chain variable region genes show that the majority of cases are hypermutated. The most representative VH family members are VH3 and VH4, in particular VH4-34 (20,21,80). IgHV-1-69 occurs most frequently in cases associated with hepatitis C virus (21% of patients) (23). These findings imply a role for the antigen in the maintenance of neoplastic B-cells.
There are no specific recurrent cytogenetic abnormalities associated with NMZL. This tumor shares gains of chromosomes 3, 7, 12, and 18, and losses of 6q23-24, as in other marginal zone lymphomas (27). Deletion of 15q25.3–q26.2 was the second most common abnormality found in NMZLs.
The 20q12 deletion is associated with the histological transformation of NMZL to diffuse large B-cell lymphoma (81).
Gene expression profiling studies revealed a molecular signature similar to that of normal marginal zone cells enriched in interleukins, integrins, PI3K, NF-KB, and TGF-b (82).
Whole exome sequencing has revealed recurrent mutations in genes involved in marginal zone differentiation, B-cell receptor signaling and chromatin conformation including KMT2D (28–34%), PTPRD (20%), NOTCH2 (20%), KLF2 (12–17%), TNFAIP3 (12%), TET2 (20%), CREBBP (20%), BRAF (16%), EZH2 (16%), and TBFRSF14 (16%) (51,83,84). The only gene mutated exclusively in NMZL is PTPRD, at a frequency of 20% (51); this is a potentially significant finding that requires confirmation.
Differential diagnosis
NMZL remains a challenging diagnosis, requiring exclusion of nodal involvement by the other types of MZL, other B-cell lymphomas, specifically follicular and lymphoplasmacytic lymphomas, and reactive conditions.
Clinical presentation is fundamental to an NMZL diagnosis; NMZL cases with splenic involvement should be carefully investigated for the possible diagnosis of SMZL. Although rare, mutations in PTPRD have been described in NMZL, but are entirely absent from SMZL (51). Nevertheless, there are still gray areas where the characteristics of nodal and SMZL cases overlap (75), and for which the clinical implications of this differential are unclear.
The differential with LPL is important because of its possible therapeutic significance. The differential of NMZL and SMZL with LPL is based on a combination of three features. LPL diagnosis is favored for cases with plasmacytic differentiation plus monoclonal paraproteinemia plus MYD88 mutations. The level of paraproteinemia is usually much higher in LPL than in MZL. Bone marrow biopsies may also be valuable, since, in contrast with the micronodular/intertrabecular pattern of infiltration by MZL, LPS usually has a more diffuse pattern, with some paratrabecular involvement and significant mastocytosis (58).
MZL and FL differ in their IHC markers, whereby MZL usually expresses MNDA and T-Bet, while being negative for BCL6, CD10 and other GC markers. When necessary, genetic and molecular studies could also contribute to the diagnosis. Use of IgVH1.2, 7q loss, KLF2, and NOTCH2 mutations point to a diagnosis of MZL.
Occasionally, NMZL may have a lymph node infiltration pattern and PD1 staining, which favor a diagnosis of peripheral T-cell lymphoma. Three patterns of staining with PD1 in NMZL have been reported: normal, follicular and diffuse. The follicular and diffuse patterns may suggest a PTCL with Tfh phenotype (85).
A monoclonal B-cell lymphocytosis diagnosis should also be considered for cases without clinical malignancy features and minimal tissue infiltration, in which the lymph node architecture remains basically undisturbed.
NMZL, like other small B-cell lymphomas, may progress to LBCL. Histological features associated with progression are the increase in the proportion of large cells, a high level of Ki67, and strong CD30 expression and EBV (53,67). Precise thresholds have not been defined, but it is advisable to measure these four features for each patient, since an increase in any of them may presage progression to LBCL.
Pediatric NMZL
There is still controversy about the existence of a pediatric type of NMZL that is distinct from pediatric follicular lymphoma. Molecular analysis of these cases reveals some common molecular alterations (86). Before making a diagnosis of NMZL in a pediatric patient, it is important to remember that these patients may show monotypic marginal zone hyperplasia, a phenomenon that has been linked to a Haemophilus influenzae-driven immune disorder (87).
Acknowledgments
Funding: This work was supported by grants from the Instituto de Salud Carlos III (ISCIII) of the Spanish Ministry of Economy and Competence (MINECO, RTICC ISCIII and CIBERONC) (SAF2013-47416-R, RD06/0020/0107-RD012/0036/0060 and Plan Nacional I+D+I: PI17/2172, PI16/01294 and PIE15/0081), AECC, and the Madrid Autonomous Community (S2017/BMD-3778).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Francesco Bertoni, Davide Rossi, Thomas Habermann, Emanuele Zucca) for the series “Marginal Zone Lymphomas” published in Annals of Lymphoma. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aol-20-17). The series “Marginal Zone Lymphomas” was commissioned by the editorial office without any funding or sponsorship. MAP declares having received lecture fees and advisory board fees from Takeda, Janssen, NanoString and Celgene. The authors have no other conflicts of interest to declare
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Schmid C, Kirkham N, Diss T, et al. Splenic marginal zone cell lymphoma. Am J Surg Pathol 1992;16:455-66. [Crossref] [PubMed]
- Matutes E, Oscier D, Montalban C, et al. Splenic marginal zone lymphoma proposals for a revision of diagnostic, staging and therapeutic criteria. Leukemia 2008;22:487-95. [Crossref] [PubMed]
- Algara P, Mateo MS, Sanchez-Beato M, et al. Analysis of the IgV(H) somatic mutations in splenic marginal zone lymphoma defines a group of unmutated cases with frequent 7q deletion and adverse clinical course. Blood 2002;99:1299-304. [Crossref] [PubMed]
- Bikos V, Darzentas N, Hadzidimitriou A, et al. Over 30% of patients with splenic marginal zone lymphoma express the same immunoglobulin heavy variable gene: ontogenetic implications. Leukemia 2012;26:1638-46. [Crossref] [PubMed]
- Fresquet V, Robles EF, Parker A, et al. High-throughput sequencing analysis of the chromosome 7q32 deletion reveals IRF5 as a potential tumour suppressor in splenic marginal-zone lymphoma. Br J Haematol 2012;158:712-26. [Crossref] [PubMed]
- Kanellis G, Roncador G, Arribas A, et al. Identification of MNDA as a new marker for Nodal Marginal Zone Lymphoma. Leukemia 2009;23:1847-57. [Crossref] [PubMed]
- Martínez N, Almaraz C, Vaque JP, et al. Whole-exome sequencing in splenic marginal zone lymphoma reveals mutations in genes involved in marginal zone differentiation. Leukemia 2014;28:1334-40. [Crossref] [PubMed]
- Mateo M, Mollejo M, Villuendas R, et al. 7q31-32 allelic loss is a frequent finding in splenic marginal zone lymphoma. Am J Pathol 1999;154:1583-9. [Crossref] [PubMed]
- Ruiz-Ballesteros E, Mollejo M, Rodriguez A, et al. Splenic marginal zone lymphoma: proposal of new diagnostic and prognostic markers identified after tissue and cDNA microarray analysis. Blood 2005;106:1831-8. [Crossref] [PubMed]
- Salido M, Baro C, Oscier D, et al. Cytogenetic aberrations and their prognostic value in a series of 330 splenic marginal zone B-cell lymphomas: a multicenter study of the Splenic B-Cell Lymphoma Group. Blood 2010;116:1479-88. [Crossref] [PubMed]
- Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: International Agency for Research on Cancer (IARC); 2008.
- Lloret E, Mollejo M, Mateo MS, et al. Splenic marginal zone lymphoma with increased number of blasts: an aggressive variant? Hum Pathol 1999;30:1153-60. [Crossref] [PubMed]
- Mollejo M, Lloret E, Menarguez J, et al. Lymph node involvement by splenic marginal zone lymphoma: morphological and immunohistochemical features. Am J Surg Pathol 1997;21:772-80. [Crossref] [PubMed]
- Franco V, Florena AM, Campesi G. Intrasinusoidal bone marrow infiltration: a possible hallmark of splenic lymphoma. Histopathology 1996;29:571-5. [Crossref] [PubMed]
- Boveri E, Arcaini L, Merli M, et al. Bone marrow histology in marginal zone B-cell lymphomas: correlation with clinical parameters and flow cytometry in 120 patients. Ann Oncol 2009;20:129-36. [Crossref] [PubMed]
- Audouin J, Le Tourneau A, Molina T, et al. Patterns of bone marrow involvement in 58 patients presenting primary splenic marginal zone lymphoma with or without circulating villous lymphocytes. Br J Haematol 2003;122:404-12. [Crossref] [PubMed]
- Parker H, McIver-Brown NR, Davis ZA, et al. CBL-MZ is not a single biological entity: evidence from genomic analysis and prolonged clinical follow-up. Blood Adv 2018;2:1116-9. [Crossref] [PubMed]
- Baseggio L, Traverse-Glehen A, Petinataud F, et al. CD5 expression identifies a subset of splenic marginal zone lymphomas with higher lymphocytosis: a clinico-pathological, cytogenetic and molecular study of 24 cases. Haematologica 2010;95:604-12. [Crossref] [PubMed]
- Falini B, Tiacci E, Pucciarini A, et al. Expression of the IRTA1 receptor identifies intraepithelial and subepithelial marginal zone B cells of the mucosa-associated lymphoid tissue (MALT). Blood 2003;102:3684-92. [Crossref] [PubMed]
- Xochelli A, Bikos V, Polychronidou E, et al. Disease-biased and shared characteristics of the immunoglobulin gene repertoires in marginal zone B cell lymphoproliferations. J Pathol 2019;247:416-21. [Crossref] [PubMed]
- Traverse-Glehen A, Davi F, Ben Simon E, et al. Analysis of VH genes in marginal zone lymphoma reveals marked heterogeneity between splenic and nodal tumors and suggests the existence of clonal selection. Haematologica 2005;90:470-8. [PubMed]
- Bikos V, Karypidou M, Stalika E, et al. An Immunogenetic Signature of Ongoing Antigen Interactions in Splenic Marginal Zone Lymphoma Expressing IGHV1-2*04 Receptors. Clin Cancer Res 2016;22:2032-40. [Crossref] [PubMed]
- Marasca R, Vaccari P, Luppi M, et al. Immunoglobulin gene mutations and frequent use of VH1-69 and VH4-34 segments in hepatitis C virus-positive and hepatitis C virus-negative nodal marginal zone B-cell lymphoma. Am J Pathol 2001;159:253-61. [Crossref] [PubMed]
- Bates I, Bedu-Addo G, Rutherford T, et al. Splenic lymphoma with villous lymphocytes in tropical West Africa. Lancet 1992;340:575-7. [Crossref] [PubMed]
- Bates I, Bedu-Addo G. Chronic malaria and splenic lymphoma: clues to understanding lymphoma evolution. Leukemia 1997;11:2162-7. [Crossref] [PubMed]
- Rinaldi A, Mian M, Chigrinova E, et al. Genome-wide DNA profiling of marginal zone lymphomas identifies subtype-specific lesions with an impact on the clinical outcome. Blood 2011;117:1595-604. [Crossref] [PubMed]
- Novak U, Rinaldi A, Kwee I, et al. The NF-{kappa}B negative regulator TNFAIP3 (A20) is inactivated by somatic mutations and genomic deletions in marginal zone lymphomas. Blood 2009;113:4918-21. [Crossref] [PubMed]
- Trøen G, Nygaard V, Jenssen TK, et al. Constitutive expression of the AP-1 transcription factors c-jun, junD, junB, and c-fos and the marginal zone B-cell transcription factor Notch2 in splenic marginal zone lymphoma. J Mol Diagn 2004;6:297-307. [Crossref] [PubMed]
- Kiel MJ, Velusamy T, Betz BL, et al. Whole-genome sequencing identifies recurrent somatic NOTCH2 mutations in splenic marginal zone lymphoma. J Exp Med 2012;209:1553-65. [Crossref] [PubMed]
- Rossi D, Trifonov V, Fangazio M, et al. The coding genome of splenic marginal zone lymphoma: activation of NOTCH2 and other pathways regulating marginal zone development. J Exp Med 2012;209:1537-51. [Crossref] [PubMed]
- Clipson A, Wang M, de Leval L, et al. KLF2 mutation is the most frequent somatic change in splenic marginal zone lymphoma and identifies a subset with distinct genotype. Leukemia 2015;29:1177-85. [Crossref] [PubMed]
- Piva R, Deaglio S, Famà R, et al. The Kruppel-like factor 2 transcription factor gene is recurrently mutated in splenic marginal zone lymphoma. Leukemia 2015;29:503-7. [Crossref] [PubMed]
- Jaramillo Oquendo C, Parker H, Oscier D, et al. Systematic Review of Somatic Mutations in Splenic Marginal Zone Lymphoma. Sci Rep 2019;9:10444. [Crossref] [PubMed]
- Trøen G, Warsame A, Delabie J. CD79B and MYD88 Mutations in Splenic Marginal Zone Lymphoma. ISRN Oncol 2013;2013:252318 [Crossref] [PubMed]
- Martinez-Lopez A, Curiel-Olmo S, Mollejo M, et al. MYD88 (L265P) somatic mutation in marginal zone B-cell lymphoma. Am J Surg Pathol 2015;39:644-51. [Crossref] [PubMed]
- Hamadeh F, MacNamara SP, Aguilera NS, et al. MYD88 L265P mutation analysis helps define nodal lymphoplasmacytic lymphoma. Mod Pathol 2015;28:564-74. [Crossref] [PubMed]
- Miranda RN, Briggs RC, Shults K, et al. Immunocytochemical analysis of MNDA in tissue sections and sorted normal bone marrow cells documents expression only in maturing normal and neoplastic myelomonocytic cells and a subset of normal and neoplastic B lymphocytes. Hum Pathol 1999;30:1040-9. [Crossref] [PubMed]
- Metcalf RA, Monabati A, Vyas M, et al. Myeloid cell nuclear differentiation antigen is expressed in a subset of marginal zone lymphomas and is useful in the differential diagnosis with follicular lymphoma. Hum Pathol 2014;45:1730-6. [Crossref] [PubMed]
- Wang Z, Cook JR. IRTA1 and MNDA Expression in Marginal Zone Lymphoma: Utility in Differential Diagnosis and Implications for Classification. Am J Clin Pathol 2019;151:337-43. [Crossref] [PubMed]
- Bob R, Falini B, Marafioti T, et al. Nodal reactive and neoplastic proliferation of monocytoid and marginal zone B cells: an immunoarchitectural and molecular study highlighting the relevance of IRTA1 and T-bet as positive markers. Histopathology 2013;63:482-98. [Crossref] [PubMed]
- Lohneis P, Wienert S, Klauschen F, et al. Marginal zone lymphomas with monocytoid morphology express T-bet and are associated with a low number of T cells in extranodal locations. Leuk Lymphoma 2014;55:143-8. [Crossref] [PubMed]
- Matutes E, Morilla R, Owusu-Ankomah K, et al. The immunophenotype of splenic lymphoma with villous lymphocytes and its relevance to the differential diagnosis with other B-cell disorders. Blood 1994;83:1558-62. [Crossref] [PubMed]
- Traverse-Glehen A, Baseggio L, Bauchu EC, et al. Splenic red pulp lymphoma with numerous basophilic villous lymphocytes: a distinct clinicopathologic and molecular entity? Blood 2008;111:2253-60. [Crossref] [PubMed]
- Mason EF, Pozdnyakova O, Li B, et al. Flow Cytometric Patterns of CD200 and CD1d Expression Distinguish CD10-Negative, CD5-Negative Mature B-Cell Lymphoproliferative Disorders. Am J Clin Pathol 2017;148:33-41. [Crossref] [PubMed]
- Curiel-Olmo S, Mondejar R, Almaraz C, et al. Splenic diffuse red pulp small B-cell lymphoma displays increased expression of cyclin D3 and recurrent CCND3 mutations. Blood 2017;129:1042-5. [Crossref] [PubMed]
- Matutes E. Immunophenotyping and differential diagnosis of hairy cell leukemia. Hematol Oncol Clin North Am 2006;20:1051-63. [Crossref] [PubMed]
- Dierlamm J, Rosenberg C, Stul M, et al. Characteristic pattern of chromosomal gains and losses in marginal zone B cell lymphoma detected by comparative genomic hybridization. Leukemia 1997;11:747-58. [Crossref] [PubMed]
- Oscier DG, Gardiner A, Mould S. Structural abnormalities of chromosome 7q in chronic lymphoproliferative disorders. Cancer Genet Cytogenet 1996;92:24-7. [Crossref] [PubMed]
- Treon SP, Xu L, Yang G, et al. MYD88 L265P somatic mutation in Waldenstrom's macroglobulinemia. N Engl J Med 2012;367:826-33. [Crossref] [PubMed]
- Traverse-Glehen A, Verney A, Gazzo S, et al. Splenic diffuse red pulp lymphoma has a distinct pattern of somatic mutations amongst B-cell malignancies. Leuk Lymphoma 2017;58:666-75. [Crossref] [PubMed]
- Spina V, Khiabanian H, Messina M, et al. The genetics of nodal marginal zone lymphoma. Blood 2016;128:1362-73. [Crossref] [PubMed]
- Martinez D, Navarro A, Martinez-Trillos A, et al. NOTCH1, TP53, and MAP2K1 Mutations in Splenic Diffuse Red Pulp Small B-cell Lymphoma Are Associated With Progressive Disease. Am J Surg Pathol 2016;40:192-201. [PubMed]
- Parry M, Rose-Zerilli MJ, Ljungstrom V, et al. Genetics and Prognostication in Splenic Marginal Zone Lymphoma: Revelations from Deep Sequencing. Clin Cancer Res 2015;21:4174-83. [Crossref] [PubMed]
- Jallades L, Baseggio L, Sujobert P, et al. Exome sequencing identifies recurrent BCOR alterations and the absence of KLF2, TNFAIP3 and MYD88 mutations in splenic diffuse red pulp small B-cell lymphoma. Haematologica 2017;102:1758-66. [Crossref] [PubMed]
- Ya-In C, Brandwein J, Pantalony D, et al. Hairy cell leukemia variant with features of intrasinusoidal bone marrow involvement. Arch Pathol Lab Med 2005;129:395-8. [PubMed]
- Waterfall JJ, Arons E, Walker RL, et al. High prevalence of MAP2K1 mutations in variant and IGHV4-34-expressing hairy-cell leukemias. Nat Genet 2014;46:8-10. [Crossref] [PubMed]
- Jiménez C, Sebastian E, Chillon MC, et al. MYD88 L265P is a marker highly characteristic of, but not restricted to, Waldenstrom's macroglobulinemia. Leukemia 2013;27:1722-8. [Crossref] [PubMed]
- Bassarova A, Troen G, Spetalen S, et al. Lymphoplasmacytic lymphoma and marginal zone lymphoma in the bone marrow: paratrabecular involvement as an important distinguishing feature. Am J Clin Pathol 2015;143:797-806. [Crossref] [PubMed]
- Ocio EM, Hernandez JM, Mateo G, et al. Immunophenotypic and cytogenetic comparison of Waldenstrom's macroglobulinemia with splenic marginal zone lymphoma. Clin Lymphoma 2005;5:241-5. [Crossref] [PubMed]
- Mollejo M, Rodriguez-Pinilla MS, Montes-Moreno S, et al. Splenic follicular lymphoma: clinicopathologic characteristics of a series of 32 cases. Am J Surg Pathol 2009;33:730-8. [Crossref] [PubMed]
- Karube K, Martinez D, Royo C, et al. Recurrent mutations of NOTCH genes in follicular lymphoma identify a distinctive subset of tumours. J Pathol 2014;234:423-30. [Crossref] [PubMed]
- Xochelli A, Kalpadakis C, Gardiner A, et al. Clonal B-cell lymphocytosis exhibiting immunophenotypic features consistent with a marginal-zone origin: is this a distinct entity? Blood 2014;123:1199-206. [Crossref] [PubMed]
- Mollejo M, Menarguez J, Guisado-Vasco P, et al. Hepatitis C virus-related lymphoproliferative disorders encompass a broader clinical and morphological spectrum than previously recognized: a clinicopathological study. Mod Pathol 2014;27:281-93. [Crossref] [PubMed]
- Martinez-Lopez A, Montes-Moreno S, Mazorra F, et al. Persistent polyclonal B-cell lymphocytosis with splenomegaly: histologic description of 2 cases. Am J Surg Pathol 2013;37:1085-90. [Crossref] [PubMed]
- Del Giudice I, Pileri SA, Rossi M, et al. Histopathological and molecular features of persistent polyclonal B-cell lymphocytosis (PPBL) with progressive splenomegaly. Br J Haematol 2009;144:726-31. [Crossref] [PubMed]
- Camacho FI, Mollejo M, Mateo MS, et al. Progression to large B-cell lymphoma in splenic marginal zone lymphoma: a description of a series of 12 cases. Am J Surg Pathol 2001;25:1268-76. [Crossref] [PubMed]
- Camacho Castañeda FI, Dotor A, Manso R, et al. Epstein-Barr virus-associated large B-cell lymphoma transformation in marginal zone B-cell lymphoma: a series of four cases. Histopathology 2020;77:112-22. [Crossref] [PubMed]
- Montalban C, Abraira V, Arcaini L, et al. Simplification of risk stratification for splenic marginal zone lymphoma: a point-based score for practical use. Leuk Lymphoma 2014;55:929-31. [Crossref] [PubMed]
- Montalbán C, Abraira V, Arcaini L, et al. Risk stratification for Splenic Marginal Zone Lymphoma based on haemoglobin concentration, platelet count, high lactate dehydrogenase level and extrahilar lymphadenopathy: development and validation on 593 cases. Br J Haematol 2012;159:164-71. [Crossref] [PubMed]
- Gruszka-Westwood AM, Hamoudi RA, Matutes E, et al. p53 abnormalities in splenic lymphoma with villous lymphocytes. Blood 2001;97:3552-8. [Crossref] [PubMed]
- Baldini L, Guffanti A, Cro L, et al. Poor prognosis in non-villous splenic marginal zone cell lymphoma is associated with p53 mutations. Br J Haematol 1997;99:375-8. [Crossref] [PubMed]
- Campos-Martín Y, Martinez N, Martinez-Lopez A, et al. Clinical and diagnostic relevance of NOTCH2-and KLF2-mutations in splenic marginal zone lymphoma. Haematologica 2017;102:e310-2. [Crossref] [PubMed]
- Karube K, Ohshima K, Tsuchiya T, et al. A "floral" variant of nodal marginal zone lymphoma. Hum Pathol 2005;36:202-6. [Crossref] [PubMed]
- Traverse-Glehen A, Bertoni F, Thieblemont C, et al. Nodal marginal zone B-cell lymphoma: a diagnostic and therapeutic dilemma. Oncology (Williston Park) 2012;26:92-9, 103-4. [PubMed]
- Berger F, Felman P, Thieblemont C, et al. Non-MALT marginal zone B-cell lymphomas: a description of clinical presentation and outcome in 124 patients. Blood 2000;95:1950-6. [Crossref] [PubMed]
- van den Brand M, van Krieken JH. Recognizing nodal marginal zone lymphoma: recent advances and pitfalls. A systematic review. Haematologica 2013;98:1003-13. [Crossref] [PubMed]
- Campo E, Miquel R, Krenacs L, et al. Primary nodal marginal zone lymphomas of splenic and MALT type. Am J Surg Pathol 1999;23:59-68. [Crossref] [PubMed]
- Inamdar KV, Medeiros LJ, Jorgensen JL, et al. Bone marrow involvement by marginal zone B-cell lymphomas of different types. Am J Clin Pathol 2008;129:714-22. [Crossref] [PubMed]
- Vroobel KM, O'Connor S, Cunningham D, et al. Florid T follicular helper cell hyperplasia associated with extranodal marginal zone lymphoma: a diagnostic pitfall which may mimic T cell lymphoma. Histopathology 2019;75:287-90. [Crossref] [PubMed]
- Camacho FI, Algara P, Mollejo M, et al. Nodal marginal zone lymphoma: a heterogeneous tumor: a comprehensive analysis of a series of 27 cases. Am J Surg Pathol 2003;27:762-71. [Crossref] [PubMed]
- Qian L, Soderquist C, Schrank-Hacker A, et al. Deletion 20q12 is associated with histological transformation of nodal marginal zone lymphoma to diffuse large B-cell lymphoma. Am J Hematol 2020;95:238-44. [Crossref] [PubMed]
- Arribas AJ, Campos-Martin Y, Gomez-Abad C, et al. Nodal marginal zone lymphoma: gene expression and miRNA profiling identify diagnostic markers and potential therapeutic targets. Blood 2012;119:e9-e21. [Crossref] [PubMed]
- Pillonel V, Juskevicius D, Ng CKY, et al. High-throughput sequencing of nodal marginal zone lymphomas identifies recurrent BRAF mutations. Leukemia 2018;32:2412-26. [Crossref] [PubMed]
- van den Brand M, Rijntjes J, Hebeda KM, et al. Recurrent mutations in genes involved in nuclear factor-kappaB signalling in nodal marginal zone lymphoma-diagnostic and therapeutic implications. Histopathology 2017;70:174-84. [Crossref] [PubMed]
- Egan C, Laurent C, Alejo JC, et al. Expansion of PD1-positive T Cells in Nodal Marginal Zone Lymphoma: A Potential Diagnostic Pitfall. Am J Surg Pathol 2020;44:657-64. [Crossref] [PubMed]
- Quintanilla-Martinez L, Sander B, Chan JK, et al. Indolent lymphomas in the pediatric population: follicular lymphoma, IRF4/MUM1+ lymphoma, nodal marginal zone lymphoma and chronic lymphocytic leukemia. Virchows Arch 2016;468:141-57. [Crossref] [PubMed]
- Kluin PM, Langerak AW, Beverdam-Vincent J, et al. Paediatric nodal marginal zone B-cell lymphadenopathy of the neck: a Haemophilus influenzae-driven immune disorder? J Pathol 2015;236:302-14. [Crossref] [PubMed]
Cite this article as: Mollejo M, Piris MA. The complex pathology and differential diagnosis of splenic and nodal marginal zone lymphoma. Ann Lymphoma 2020;4:18.


