
Pathology of extranodal marginal zone lymphoma at different anatomic sites
Mucosa associated lymphoid tissue (MALT)
Within the body there are areas of organised lymphoid tissue within tissues that are not considered primary lymphoid organs. This lymphoid tissue is mostly found in the gastrointestinal tract (gut-associated lymphoid tissue, GALT) with a particular condensation in the terminal ileum in the form of Peyer’s patches. In this location the lymphoid tissue has a specific relationship with the overlying epithelium. In the surface of the dome epithelium overlying the lymphoid tissue are specially adapted M-cells that facilitate the transfer of large molecules from the gut lumen for presentation to the immune system. Similar aggregates of lymphoid tissue are encountered in the lung (Bronchus-associated lymphoid tissue, BALT) and the skin (skin-associated lymphoid tissue, SALT).
This organised tissue, termed MALT, is exemplified by the organised lymphoid tissue in the gut and is considered to include not only the organised lymphoid follicle but also the peri-follicular lymphocytes and plasma cells in the lamina propria, the intraepithelial B cells present in the dome epithelium and the mesenteric lymph nodes. The follicles in both the Peyer’s patches and the mesenteric lymph nodes have a well developed marginal zone external to the mantle zone (Figure 1). This compartment is not well developed in other lymph node groups but is seen in the spleen. It is thought this is related to areas that are associated with exposure to significant amounts of external antigen.
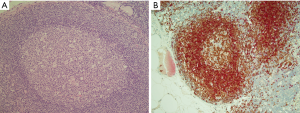
Structurally the lymphoid tissue in the gut mucosa consists of a lymphoid follicle with a reactive germinal centre that has the dark zone at the base with the light zone closest to the mucosal surface (Figure 2A). The germinal centre is surrounded by a well formed mantle zone that contains predominantly IgD positive naïve B cells and which is thickest below the surface epithelium and is rather thin where it abuts the muscularis mucosae (Figure 2B). External to this is the marginal zone that consists predominantly of slightly larger B cells with more abundant pale cytoplasm. There are other cell types in the marginal zone including plasma cells, T cells, macrophages and dendritic cells. This zone is also more developed below the surface where epithelial crypts are absent. The marginal zone abuts the overlying dome epithelium with infiltration of the epithelium by marginal zone B cells forming a lymphoepithelium (Figure 2A,C).
Extranodal marginal zone lymphoma of MALT (malt lymphoma)
Isaacson and Wright were the first to recognise a group of lymphomas that arose in extranodal sites and which appeared to have characteristic clinico-pathological characteristics (1,2). In recognition that these occurred predominantly in the gastrointestinal tract, particularly in the stomach, and that the organisational features of the lymphoid infiltrate resembled that of the Peyer’s patch these lymphomas became termed MALT lymphomas. Subsequent to this first description similar lymphomas have been recognised in almost every extranodal site in the body. The only site where MALT lymphoma is not encountered is in the area of highest quantity of constitutive GALT, the terminal ileum. It appears that acquisition of organised lymphoid tissue with the components similar to those of the mucosal compartment of GALT is the crucial first step in the development of MALT lymphoma. The stimulus to the acquisition of MALT varies between organs with some developing in response to a local prolonged low grade antigen stimulation associated with a generally indolent bacterial infection while others develop in the context autoimmune disease (3). At many sites the underlying prodromal event remains unclear. Notwithstanding the underlying pathogenetic pathway the resultant MALT lymphomas show remarkably similar histopathological features. Detailed morphological, immunohistochemical and molecular studies together with in-vitro experiments have indicated that the normal counterpart of the neoplastic cell of MALT lymphoma is the marginal zone B cell of the acquired MALT (4).
In keeping with the normal behaviour of marginal zone B cells the neoplastic cells retain the ability to recirculate through the body relocating to the mucosa through interaction with appropriate cell adhesion molecules (5,6). In the stomach it has been clearly shown that this results in multifocal disease with neoplastic cells from the main bulk of the tumour found in the marginal zone/peri-follicular zones of lymphoid tissue elsewhere in the gastric mucosa, sometimes forming micro-lymphomas around a single follicle (7,8). This recirculation to other areas of MALT may explain multifocality in other organs, the tendency for ipsilateral involvement in paired organs such as salivary glands and the risk of spread to other extranodal sites rather than peripheral lymph nodes.
Histology of malt lymphomas
MALT lymphoma cells are initially located in the region of the peri-follicular marginal zone. This infiltrate expands to occupy the surrounding tissue to efface the surrounding stroma. While residual remnants of the acquired lymphoid tissue are usually present in the form of all or parts of reactive germinal centres in established MALT lymphoma these are frequently overrun.
The neoplastic cells of MALT lymphoma have a variable morphological appearance both between cases in different cases but also within any individual lymphoma deposit. The original descriptions recognised a cell with rather scanty cytoplasm and irregular nuclei with dense chromatin and no nucleolus (1,2) (Figure 3). The resemblance of this cell type to the small cells in the germinal centre led to the use of the term centrocyte-like (CCL) cell. In addition to CCL cells the cells may resemble monocytoid B cells with more abundant pale cytoplasm, well demarcated cytoplasmic borders and round nuclei that lack nucleoli while other cells show plasmacytoid features. A degree of plasmacytic differentiation is seen in many cases but is more pronounced in approximately a third of cases although this may rise to 40% in MALT lymphomas of the ocular region (9,10). In some cases the plasma cell component may be sufficiently extreme to almost completely eclipse the small B cell component raising a differential diagnosis with plasmacytoma. Cells with Dutcher bodies may be seen and in some instances the presence of periodic acid schiff (PAS) positive IgM-type Dutcher bodies may be useful in the distinction between MALT lymphoma and plasmacytoma in aspiration cytology preparation (11). Deposition of amyloid and light chain disease has been associated with MALT lymphoma with this a more frequent occurrence in lymphomas involving the lung (12-17). Amyloid associated with MALT lymphoma is more frequently of nodular rather than systemic type and may be in a peri-tumoural location (14-16). Up to 80% of pulmonary light chain deposition disease has been associated with MALT lymphoma with 50% of these patients having associated Sjogren’s syndrome (17). Rare cases of MALT lymphoma have also been associated with crystal storing histiocytosis (18,19).
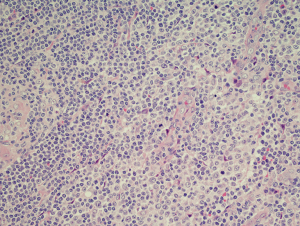
Scattered large transformed/activated B cells are a constant feature but are not seen in clusters or groups. These cells have abundant cytoplasm and large nuclei that have open chromatin with eosinophilic nucleoli. Significant clusters of large cells are not seen and if present should raise the possibility of progression to diffuse large B cell lymphoma.
Lymphoepithelial lesions
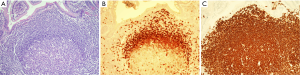
Although termed MALT lymphomas due to the overall resemblance to the organised lymphoid tissue found at extranodal sites, predominantly mucosal, not all MALT lymphomas arise in true mucosae and many arise at sites devoid of epithelial structures such as soft tissue. However, when epithelial structures are present the neoplastic interact with them in a way that partly recapitulates the lymphoepithelium of the Peyer’s patch. In contrast to the single B cells seen in the dome epithelium the neoplastic marginal zone B cells infiltrate in clusters and exert a destructive/toxic effect on the epithelial cell and these structures are termed lymphoepithelial lesions (LELs) (20). The interaction between the neoplastic lymphocytes and the epithelial structures varies between sites. LELs in the stomach show infiltration of the epithelium by groups of three or more neoplastic cells (21). With time the epithelial cells become enlarged with cytoplasm that becomes more eosinophilic (Figure 4). The is due to initial swell if intracellular organelles which subsequently are destroyed. Eventually the epithelial structure is lost although in some instances isolated endocrine cells may remain (22). In salivary glands the LELs resemble the epi-myoepithelial lesions of myoepithelial sialadenitis (23) while in the thyroid the neoplastic marginal zone cells frequently fill and expand the thyroid follicles (24) (Figure 5). Cyst formation in the lungs has been described in association with MALT lymphoma of the thymus (25,26).
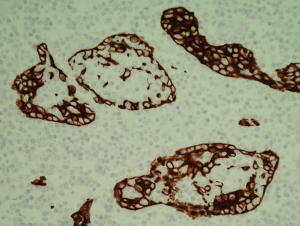
While initially thought to be pathognomonic for MALT lymphoma it is now recognised that the LELs are not essential for a diagnosis even in mucosal locations nor are they specific. Identical structures can be encountered in reactive infiltrates such as rheumatoid lung disease and in association with other histologically low grade B cell lymphoma (Figure 6), particularly in follicular lymphoma of the thyroid but also with follicular lymphomas at other sites and in mantle cell lymphoma involving the gastrointestinal tract.
Follicular colonisation
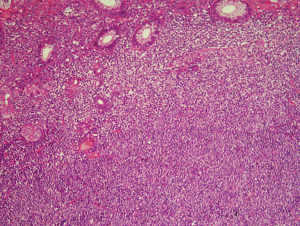
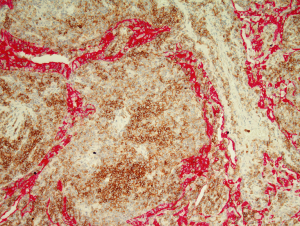
Lymphoid follicles are a ubiquitous feature in MALT lymphoma, being a constant component of the organised lymphoid tissue that is a pre-requisite for the development of the lymphoma. While in many cases the follicles are largely or completely effaced by the neoplastic infiltrate there are some instances where the neoplastic cells specifically colonised by the neoplastic cells leaving the underlying follicular structure intact (27). This is a recapitulation of the normal response of reactive marginal zone B cells under the influence of antigen exposure (28).
The cell morphology in the colonised follicles varies between cases. In some cases there is a mixture of cells similar to that seen in follicular lymphoma (27). Other cases have intrafollicular cells with a more activated cellular appearance that are larger than the extra-follicular component, while some cases appear to have an intra-follicular component that is mainly composed of plasma cells (27). In some cases the colonisation may be sufficient to make the distinction between follicular lymphoma and MALT lymphoma challenging (27) (Figure 7). This is particularly true in small or distorted biopsies that frequently are derived from delicate sites such as the peri-ocular region. This problematic differential can be further compounded by the fact that the neoplastic marginal zone B cells have the potential to up-regulate germinal centre associated markers such as CD10 and bcl-6 protein.
Immunophenotype
The immunophenotype of MALT lymphoma is not specific. There is expression of pan B cell markers CD19, CD20, CD22 and CD79a. The neoplastic cells also express CD27, a marker of activated and memory B cells. The plasma cell component can be highlighted with an antibody to CD138. There is expression of immunoglobulin which may be easier to demonstrate in cases showing plasmacytoid or plasmacytic differentiation. Expression of IgM heavy chain is most frequent with some expressing IgA while IgG expression is rare (21). There is light chain restriction. Approximately 30–50% of cases express CD43 (29) and this may be helpful in distinguishing MALT lymphoma from reactive infiltrates. Expression of CD5 is seen in 5–10% of cases (30) but these lack expression of CD23 and cycninD1 distinguishing the infiltrate from other CD5 positive B cell lymphomas. Cases with expression of CD5 appear to have a more aggressive course with higher potential for dissemination, including bone marrow infiltration, and for early relapse (30). Many CD5 positive cases arise in the orbital/peri-orbital area (30). While the neoplastic cells of MALT lymphoma may upregulate CD10 and bcl-6 protein in the germinal centre the extra-follicular component is negative for these markers. There is expression of bcl-2 protein.
Proliferation is low consistent with an indolent B cell lymphoma. Staining for cytokeratin may be useful in highlighting lymphoepithelial lesions. Staining for follicular dendritic cells (CD21 and/or CD23) highlights residual meshworks in cases where the follicular structures have been entirely effaced. Staining for IgD can be used to highlight the mantle zone B cells and is particularly useful in identifying subtle expansion of the marginal zone in the early MALT lymphoma particularly those arising in the salivary gland and thyroid.
Immunoproliferative small intestinal disease
Immunoproliferative small intestinal disease (IPSID) is a particular type of MALT lymphoma first described by Ramot in 1965 (31). The disease has a distinctive geographic distribution being found most commonly around the Mediterranean area, the Middle East and the Cape area of South Africa (32). Histologically the disease is characterised by extreme plasmacytic differentiation. The villi are broad but not shortened. Three stages are recognised (33). In stage A the infiltrate is confined to the mucosa and mesenteric lymph nodes. In stage B the infiltrate extends beyond the muscularis mucosae and in stage C there is progression to lymphoid masses with or without transformation. In stage A the B cell component is usually very scanty and may only be present around the crypts forming lymphoepithelial lesions which can be difficult to identify without the use of an anti-cytokeratin antibody. With progression to stage B and C the B cell component becomes more prominent. The immunohistochemical characteristic of this disorder is the production of IgA without light chain. The heavy chain is IgA1 although a minority of cases show both IgA1 and IgA2 (34).
Precursor lesions
The background tissue in most cases will show evidence of the predisposing disorder such as Hashimoto’s thyroiditis in the thyroid or Sjogren’s syndrome in salivary glands. Where an infective organism is implicated then this can either be demonstrated by a direct method in the tissue such as histochemical or immunohistochemical staining of Helicobacter pylori on gastric mucosa. In ocular adnexal lymphoma Chlamydia (also sometimes referred to as chlamydophilia) psittaci can be detected by immunohistochemical or PCR based techniques from formalin fixed paraffin embedded material (35). In other cases appropriate serological studies are recommended such as for Borrelia burgdorferi in cutaneous MALT lymphoma. In gastric MALT lymphoma cases the organism is sometimes to scanty to demonstrate histologically or has been eradicated prior to biopsy and in these cases serological assessment is necessary to detect low organism numbers or prior infection, as antibodies may remain detectable for up to 2 years following eradication therapy (36). Helicobacter associated gastric MALT lymphoma is most frequently located in the antrum or distal stomach as this is where the acquired lymphoid tissue is most pronounced. In recent years the level of association between gastric MALT lymphoma and Helicobacter pylori has dropped significantly. In the United Kingdom this association has dropped from an initial reported rate of 92% (37) to a level in the region of 30% (38). This may have implications for the location of the lymphoma in the stomach with more tumours presenting more proximally in the gastric wall.
Recently an association between MALT lymphoma and IgG4 related disease (IgG4-RD) has been proposed (39-41). Any association remains uncertain but it has been suggested that there may be an increased risk of developing lymphoma in patients with IgG4-RD while it has also been suggested that MALT lymphoma may predispose to the development of IgG4-RD (39,42,43). Most of these cases arise in the ocular region (39) and have been described in patients in the East Asia (40,41,44). One report suggested that there is a group of primary cutaneous MALT lymphomas that show plasmacytic differentiation are frequently associated with IgG4 expression (45).
Differential diagnosis
The principle differential diagnoses lie on the one hand with the pre-existing/underlying reactive proliferation from within which the lymphoma might develop and on the other hand with other histologically low grade B-cell lymphomas. More recently it has been recognised that MALT lymphoma may be associated with a striking infiltrate of T cells with a T follicular helper phenotype which may be sufficient to raise the possibility on angioimmunoblastic T cell lymphoma (46).
Reactive vs. neoplastic MALT
This distinction may be problematic in small biopsies from any extranodal site and repeated biopsies may be necessary to achieve a confident diagnosis. In the stomach the Wotherspoon score was developed to indicate the degree of certainty for a diagnosis of lymphoma (47) with the higher scores (scores 4 & 5) being associated with the presence of clonal populations detected by molecular techniques while lower scores were not associated with clonal populations (48).
The earliest morphological indication for the development of a neoplastic population is an expansion of the marginal zone component of the acquired MALT. In the stomach this is characterised by infiltration of small B cells into the more superficial parts of the mucosa, surrounding the glands with an irregular periphery. In the thyroid and the salivary gland the earliest change is expansion of the marginal zone between the follicle mantles and the epithelial structures resulting in a zone of cells with monocytoid appearance that appears pale at low power external to the mantle (23,24).
Immunohistochemical staining for light chain expression may be helpful but interpretation can be problematic in small or distorted biopsies. Aberrant expression of CD43, if present, is helpful in identifying a neoplastic population. Staining for IgD, while not identifying the lymphoma cells, can be helpful in defining the mantle zone and distinguishing these cells from any neoplastic component exterior to the mantle.
Clonality studies, when applied with the BIOMED 2 primer set, give a high rate of detection of a clonal population. False positive results are rare but there is potential for false negative results due to the ongoing somatic mutation in the immunoglobulin genes that are seen in MALT lymphoma (49). This should be reduced if all the primer sets are included in the study.
Atypical marginal zone hyperplasia mimics MALT lymphoma (50). This condition is encountered mainly in children at ages when MALT lymphoma is rare. In addition to the morphological similarities the atypical marginal zone cells aberrantly express CD43 and show light chain restriction (always lambda-type) (50). Distinct from MALT lymphoma the atypical marginal zone cells do not express CD27 (50). Cases of atypical marginal zone hyperplasia, while showing monotypic light chain restriction, are always polyclonal at the genetic level (50) and appropriate clonality studies can confidently distinguish between this condition and MALT lymphoma.
MALT vs. other indolent B cell lymphoma.
Usually this distinction is not a significant problem if appropriate immunohistochemical studies are undertaken. Mantle cell lymphoma can be distinguished from MALT lymphoma by the application of antibodies to cyclinD1 and SOX11, particularly in cases with expression of CD43 or CD5. The distinction with follicular lymphoma may be more problematic, particularly is small or crushed biopsies. MALT lymphomas with follicular colonisation can resemble true follicular lymphoma. While application of antibodies to CD10 and bcl-6 protein may help distinguish the two the possibility of upregulation of these germinal centre related proteins by neoplastic marginal zone cells when they relocate to the follicle centres can make the distinction difficult. In these instances careful assessment of the extra-follicular component may be helpful as, while neoplastic follicle centre cells down-regulate expression of CD10 and bcl-6 in the interfollicular compartment MALT lymphoma cells in this area do not express germinal centre related proteins. Fluorescent in-situ hybridisation (FISH) studies for the translocations t(14;18)(q32;q21) and/or t(11;18)(q21;q21) may help in the distinction between follicular and MALT lymphoma but neither is found in all cases of the respective lymphoma. In addition the t(14;18)(q32;q21) [IGH-BCL2 fusion] found in follicular lymphoma must be distinguished from the t(14;18)(q32;q21) [IGH-MALT1] found in some MALT lymphomas, particularly in the ocular and gastric context.
Assessment of gastric biopsies following helicobacter eradication for MALT lymphoma
Following Helicobacter eradication in the context gastric MALT lymphoma regular follow up endoscopy is advised (36). An initial biopsy is recommended to assess for successful eradication. This and subsequent endoscopies are also essential as the only way to assess for response. Time to remission is very variable and may take up to 5 years (unpublished data). Reporting of sequential biopsies should not only be used to assess for the presence or absence of complete response but can also inform as to whether the lymphoma is responding in a more gradual way. This can prevent the application of unnecessary and more aggressive intervention such as chemotherapy or radiotherapy. In order to give a temporal comparison in sequential biopsies The GELA group devised a reporting system to describe the appearances in post-eradication biopsies (51). This assessed the presence of cellular density and the presence of stromal changes to give a spectrum of appearances from “no change” to “complete remission”. Between these extremes two other groups were identified. Cases with “responding disease” showed reduction of the cellular infiltrate with fibrosis of the lamina propria indication some regression of disease and suggesting no further intervention was necessary as the regressive changes could continue. A group a cases in which there were residual nodular lymphoid aggregates were labelled “minimal residual disease”. These are innocuous infiltrates with low risk of progression to significant recurrent disease, but sensitive molecular studies have shown that these may harbour residual clonal lymphoma cells. This GELA scheme has been shown to be clinically useful and reproducible between pathologists (52,53).
Over time follow up biopsies may identify relapses that may be detected either on routine histology or by molecular studies (54,55). Some of these will be associated with Helicobacter reinfection and may respond to further eradication therapy. Others may be persistent and require additional intervention but a proportion will transient with spontaneous regression (54,55).
Transformation
Transformation of MALT lymphoma to diffuse large B cell lymphoma is less frequent than is seen in follicular lymphoma, occurring in 3–12% of cases (56,57). While many are of non-germinal centre/activated B cell type a significant number have a germinal centre phenotype while being clonally related to the underlying MALT lymphoma at the genetic level (58,59).
The criteria for the diagnosis of transformation are vague and controversial (60,61). While the presence of sheets of neoplastic large cells is not a significant diagnostic issue, the lower cut off for the diagnosis of a sheet of cells in early transformation is vague. A large cell component of up to 10% of total tumour cells or clusters of to 20 large cells has been suggested as significant finding with an outcome that is worse than for standard pure low grade cases and large cell infiltrates greater than this have been considered to be a diffuse large B cell component (61). Crucial to the assessment of transformation is the exclusion of the possibility that the large cell clusters are not residual reactive germinal cells in partially collonised or partly overrun follicles. This can be achieved by application of antibodies against germinal centre cell related antigens (CD10, bcl-6 protein), lack of expression of bcl-2 protein and the presence of follicular dendritic cells associated with the large cells.
Conclusions
Extranodal marginal zone lymphomas of MALT have been described in almost all locations in the body with possible exception of the terminal ileum. They arise in acquired lymphoid tissue that has usually undergone prolonged antigen stimulation. The cause of the development of MALT varies from site to site but where this has been ascertained is usually the result of chronic infection or an autoimmune disorder.
Morphologically and immunophenotypically MALT lymphomas are very similar at all sites with minor variation in the degree of plasmacytic/cytoid differentiation, the appearance of lymphoepithelial lesions and expression of CD5.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Francesco Bertoni, Davide Rossi, Thomas Habermann, Emanuele Zucca) for the series “Marginal Zone Lymphomas” published in Annals of Lymphoma. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aol-20-14). The series “Marginal Zone Lymphomas” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Isaacson P, Wright DH. Malignant lymphoma of mucosa-associated lymphoid tissue. A distinctive type of B-cell lymphoma. Cancer 1983;52:1410-6. [Crossref] [PubMed]
- Isaacson P, Wright DH. Extranodal malignant lymphoma arising from mucosa-associated lymphoid tissue. Cancer 1984;53:2515-24. [Crossref] [PubMed]
- Zucca E, Bertoni F. The spectrum of MALT lymphoma at different sites: biological and therapeutic relevance. Blood 2016;127:2082-92. [Crossref] [PubMed]
- Ortiz-Hidalgo C, Wright DH. The morphological spectrum of monocytoid B-cell lymphoma and its relationship to lymphomas of mucosa associated lymphoid tissue. Histopathology 1992;21:555-61. [Crossref] [PubMed]
- Hamann A, Andrew DP, Jablonski-Westrich D, et al. Role of α4-integrins in lymphocyte homing to mucosal tissues in vivo. J Immunol 1994;152:3282-93. [PubMed]
- Dogan A, Du M, Koulis K, et al. Expression of lymphocyte homing receptors and vascular addressins in low-grade B-cell lymphomas of mucosa-associated lymphoid tissue. Am J Pathol 1997;151:1361-9. [PubMed]
- Wotherspoon AC, Doglioni C, Isaacson PG. Low-grade gastric B-cell lymphoma of mucosa-associated lymphoid tissue (MALT): A multifocal disease. Histopathology 1992;20:29-34. [Crossref] [PubMed]
- Du MQ, Diss TC, Dogan A, et al. Clone-specific PCR reveals wide dissemination of gastric MALT lymphoma to the gastric mucosa. J Pathol 2000;192:488-93. [Crossref] [PubMed]
- Molina TJ, Lin P, Swerdlow SH, et al. Marginal zone lymphomas with plasmacytic differentiation and related disorders. Am J Clin Pathol 2011;136:211-25. [Crossref] [PubMed]
- Coupland SE, Damato B. Lymphomas involving the eye and the ocular adnexa. Curr Opin Ophthalmol 2006;17:523-31. [PubMed]
- Nasu A, Igawa T, Sato H, et al. Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue with plasma cell differentiation: periodic acid-schiff reaction positive Dutcher body is a diagnostic clue to distinguish it from plasmacytoma. Diagn Cytopathol 2017;45:547-51. [Crossref] [PubMed]
- Utz JP, Swensen SJ, Gertz MA. Pulmonary amyloidosis. The Mayo Clinic experience from 1980 to 1993. Ann Intern Med 1996;124:407-13. [Crossref] [PubMed]
- Grogg KL, Aubr MC, Vrana JA, et al. Nodular pulmonary amyloidosis is characterised by localised immunoglobulin deposition and is frequently associated with an indolent B-cell lymphoproliferative disorder. Am J Surg Pathol 2013;37:406-12. [Crossref] [PubMed]
- Ryan RJ, Sloan JM, Collins AB, et al. Extranodal marginal zone lymphoma of mucosa associated lymphoid tissue with amyloid deposition: a clinicopathologic case series. Am J Clin Pathol 2012;137:51-64. [Crossref] [PubMed]
- Telio D, Bailey D, Chen C, et al. Two distinct syndromes of AL amyloidosis: a case series and review of the literature. Am J Hematol 2010;85:805-8. [Crossref] [PubMed]
- Filippi N, Diotti C, Donghi SM, et al. Diagnostic and therapeutic implications of pulmonary lymphoma associated with nodular amyloidosis. Ann Thorac Surg 2019;107:e325-7. [Crossref] [PubMed]
- Baqir M, Moua T, White D, et al. Pulmonary nodular and cystic light chain deposition disease: a retrospective review of 10 cases. Respir Med 2020;164:105896 [Crossref] [PubMed]
- Balakrishna JP, Jaffe ES. Crystal-storing histiocytosis associated with thymic extranodal marginal zone lymphoma. Blood 2017;130:1683. [Crossref] [PubMed]
- Kokuho N, Terasaki Y, Kunugi S, et al. Localised pulmonary crystal-storing histiocytosis complicating pulmonary mucosa-associated lymphoid tissue lymphoma presenting with multiple mass lesions. Hum Pathol 2017;65:180-6. [Crossref] [PubMed]
- Papadaki L, Wotherspoon AC, Isaacson PG. The lymphoepithelial lesion of gastric low-grade B-cell lymphoma of mucosa-associated lymphoid tissue (MALT): an ultrastructural study. Histopathology 1992;21:415-21. [Crossref] [PubMed]
- Cook JR, Isaacson PG, Chott A, et al. Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). In: Swerdlow SH, Campo E, Harris NL, et al. editors. WHO classification of tumours of haemopoietic and lymphoid tissues. Lyon: IARC, 2017:259-62.
- Sutak J, Stoddard C, Smith ME. Solitary epithelial cells in B cell gastric MALT lymphoma. J Clin Pathol 2005;58:1226-8. [Crossref] [PubMed]
- Hyjek E, Smith WJ, Isaacson PG. Primary B-cell lymphoma of the salivary glands and its relationship to myoepithelial sialadenitis. Hum Pathol 1988;19:766-76. [Crossref] [PubMed]
- Hyjek E, Isaacson PG. Primary B cell lymphoma of the thyroid and its relationship to Hashimoto’s thyroiditis. Hum Pathol 1988;19:1315-26. [Crossref] [PubMed]
- Kang LY, Ho SP, Chou YP. Primary thymic mucosa-associated lymphoid tissue lymphoma with multiple thin walled lung cysts: case report and literature review. Chin J Cancer Res 2013;25:354-357. [PubMed]
- Arai H, Tajiri M, Kaneko S, et al. Two surgical cases of thymic MALT lymphoma associated with multiple lung cysts: possible association with Sjogren’s syndrome. Gen Thorac Cardiovasc Surg 2017;65:229-34. [Crossref] [PubMed]
- Isaacson PG, Wotherspoon AC, Diss TC, et al. Follicular colonization in B-cell lymphoma of mucosa associated lymphoid tissue. Am J Surg Pathol 1991;15:819-28. [Crossref] [PubMed]
- Cerutti A, Cols M, Puga I. Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nat Rev Immunol 2013;13:118-32. [Crossref] [PubMed]
- Zhang Q, Pocrnich C, Kurain A, et al. Amyloid deposition in extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue: A clinicopathologic study of 5 cases. Pathol Res Pract 2016;212:185-9. [Crossref] [PubMed]
- Ferry JA, Yang WI, Zukerberg LR, et al. CD5+ extranodal marginal zone B-cell (MALT) lymphoma. A low grade neoplasm with a propensity for bone marrow involvement and relapse. Am J Clin Pathol 1996;105:31-7. [Crossref] [PubMed]
- Ramot B, Shahin N, Bubiss JJ. Malabsorption syndrome in lymphoma of the small intestine. A study of 13 cases. Isr J Med Sci 1965;1:221-6. [PubMed]
- Price SK. Immunoproliferative small intestinal disease. A study of 13 cases with alpha heavy chain disease. Histopathology 1990;17:7-17. [Crossref] [PubMed]
- Galian A, Lecestre MJ, Scotto J, et al. Pathological study of alpha-chain disease, with special emphasis on evolution. Cancer 1977;39:2081-101. [Crossref] [PubMed]
- Isaacson PG, Dogan A, Price SK, et al. Immunoproliferative small-intestinal disease: an immunohistochemical study. Am J Surg Pathol 1989;13:1023-33. [Crossref] [PubMed]
- Ponzoni M, Ferreri AJM, Guidoboni M, et al. Chlamydia infection and lymphomas: association beyond ocular adnexal lymphomas highlighted by multiple detection methods. Clin Cancer Res 2008;14:5794-800. [Crossref] [PubMed]
- Zucca E, Arcaini L, Buske C, et al. Marginal zone lymphomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2020;31:17-29. [Crossref] [PubMed]
- Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, et al. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet 1991;338:1175-6. [Crossref] [PubMed]
- Sena Teixeira Mendes L. D Attygalle A, C Wotherspoon A. Helicobacter pylori infection in gastric extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT) lymphoma: a re-evaluation. Gut 2014;63:1526-7. [Crossref] [PubMed]
- Ferry JA. IgG4-related lymphadenopathy and IgG4-related lymphoma: moving targets. Diagn Histopathol 2013;19:128-39. [Crossref]
- Cheuk W, Yuen HKL, Chan ACL, et al. Ocular adnexal lymphoma associated with IgG4+ chronic sclerosing dacryadenitis: a previously undescribed complication of IgG4-related sclerosing disease. Am J Surg Pathol 2008;32:1159-67. [Crossref] [PubMed]
- Sato Y, Ohshima KI, Ichimura K, et al. Ocular adnexal IgG4-related disease has uniform clinicopathology. Pathol Int 2008;58:465-70. [Crossref] [PubMed]
- Takahashi N, Ghazale AH, Smyrk TC, et al. Possible association between IgG4-associated systemic disease with or without autoimmune pancreatitis and non-Hodgkin lymphoma. Pancreas 2009;38:523-6. [Crossref] [PubMed]
- Yamamoto M, Takahashi H, Tabeya T, et al. Risk of malignancies in IgG4-related disease. Mod Rheumatol 2012;22:414-8. [Crossref] [PubMed]
- Kubota T, Moritani S, Yoshino T, et al. Ocular adnexal marginal zone B cell lymphoma infiltrated by IgG4 positive plasma cells. J Clin Pathol 2010;63:1059-65. [Crossref] [PubMed]
- Brenner I, Roth S, Puppe B, et al. Primary cutaneous marginal zone lymphomas with plasmacytic differentiation show frequent IgG4 expression. Mod Pathol 2013;26:1568-76. [Crossref] [PubMed]
- Vroobel KM, O’Connor S, Cunningham D, et al. Florid T follicuolar helper cell hyperplasia associated with extranodal marginal zone lymphoma: a diagnostic pitfall which may mimic T cell lymphoma. Histopathology 2019;75:287-90. [Crossref] [PubMed]
- Wotherspoon AC, Doglioni C, Diss TC, et al. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet 1993;342:575-7. [Crossref] [PubMed]
- Hummel M, Oeschger S, Barth TFE, et al. Wotherspoon criteria combined with B cell clonality analysis by advanced polymerase chain reaction technology discriminates covert gastric marginal zone lymphoma from chronic gastritis. Gut 2006;55:782-7. [Crossref] [PubMed]
- Lu C, He Q, Zhu W, et al. The value of detecting immunoglobulin gene rearrangements in the diagnosis of B-cell lymphoma. Oncotarget 2017;8:77009. [Crossref] [PubMed]
- Attygalle AD, Liu H, Shirali S, et al. Atypical marginal zone hyperplasia of mucosa-associated lymphoid tissue: a reactive condition of childhood showing immunoglobulin lambda light-chain restriction. Blood 2004;104:3343-8. [Crossref] [PubMed]
- Copie-Bergman C, Gaulard P, Lavergne-Slove A, et al. Proposal for a new histological grading system for post-treatment evaluation of gastric MALT lymphoma. Gut 2003;52:1656. [Crossref] [PubMed]
- Shiozawa E, Norose T, Kaneko K, et al. Clinicopathological comparison of the World Health Organisation/Wotherspoon score to the Groupe d’Etude des Lymphomes de l’Adult grade for the post-treatment evaluation of gastric mucosa-associated lymphoid tissue lymphoma. J Gastroenterol Hepatol 2009;24:307-15. [Crossref] [PubMed]
- Copie-Bergman C, Wotherspoon AC, Capella C, et al. Gela histological scoring system for post-treatment biopsies of patients with gastric MALT lymphoma is feasible and reliable in routine practice. Br J Haematol 2013;160:47-52. [Crossref] [PubMed]
- Montalban C, Manzanal A, Boixeda D, et al. Helicobacter pylori eradication for the treatment of low-grade gastric MALT lymphoma: follow-up together with sequential molecular studies. Ann Oncol 1997;8:37-9. [Crossref] [PubMed]
- Bertoni F, Conconi A, Capella C, et al. Molecular follow-up in gastric mucosa-associated lymphoid tissue lymphomas: early analysis of the LY03 cooperative trial. Blood 2002;99:2541-4. [Crossref] [PubMed]
- Zucca E, Conconi A, Laszlo D, et al. Addition of rituximab to chlorambucil produces superior event-free survival in the treatment of patients with extranodal marginal zone B-cell lymphoma: 5-year analysis of the IELSG-19 randomised study. J Clin Oncol 2013;31:565-72. [Crossref] [PubMed]
- Conconi A, Franceschetti S, Aprile von Hohenstaufen K, et al. Histologic transformation in marginal zone lymphomas. Ann Oncol 2015;26:2329-35. [Crossref] [PubMed]
- Maeshima AM, Taniguchi H, Toyoda K, et al. Clinicopathological features of histological transformation from extranodal marginal zone B-cell lymphoma to diffuse large B-cell lymphoma: an analysis of 467 patients. Br J Haematol 2016;174:923-31. [Crossref] [PubMed]
- Gorodetskiy VR, Probatova NA, Radenska-Lopovok SG, et al. Clonal relationship of marginal zone lymphoma and diffuse large B-cell lymphoma in Sjogren’s syndrome patients: case series study and review of the literature. Rheumatol Int 2020;40:499-506. [Crossref] [PubMed]
- de Jong D, Boot H, van Heerde P, et al. Histological grading in gastric lymphoma: pretreatment criteria and clinical relevance. Gastroenterology 1997;112:1466-74. [Crossref] [PubMed]
- de Jong D, Boot H, Taal B. Histological grading with clinical relevance in gastric mucosa-associated lymphoid tissue (MALT) lymphoma. Recent Results Cancer Res 2000;156:27-32. [Crossref] [PubMed]
Cite this article as: Wotherspoon A. Pathology of extranodal marginal zone lymphoma at different anatomic sites. Ann Lymphoma 2020;4:15.


