
Primary vitreoretinal lymphoma: recent advances and literature review
Introduction
Primary vitreoretinal lymphoma (PVRL) is a rare form of intraocular malignancy, considered a particular subset of central nervous system (CNS) lymphoma. PVRL represents one of the most challenging ocular conditions to manage for the clinician, due to the lack of standardized guidelines for diagnosis, treatment and follow-up (1-6). Furthermore, due to its rarity, it is often misdiagnosed, resulting in several months or years of diagnostic delay (7). Clinical findings are rarely specific, masquerading as posterior or intermediate uveitis of other etiologies. However recently, novel eye imaging modalities like widefield color fundus photography, fluorescein angiography (FA) and optical coherence tomography (OCT) have allowed to identify typical lymphoma features. Indeed, identifying early signs of PVRL is of paramount importance to decrease the likely of CNS involvement which account for the high mortality rate of the disease (1,5-7).
Diagnosis of certainty requires invasive vitreoretinal biopsy. However, cytology specimens have been shown to generate false negative results in a consistent percentage of analyses. The causes for such a high rate of false negatives include the paucity and fragility of lymphomatous cells in the sample, and the cytopathologist experience (8). Recently, new techniques have been established to increase the predictive value of PVRL diagnosis. One of them is the detection of the mutation L265P in the gene MYD88 (highly specific for large B cells lymphomas) in ocular fluids like the aqueous and vitreous humor, which has represented a useful tool in the identification of PVRL (9).
Also, therapy regimens pose a significant challenge, as PVRL is likely to spread to CNS during the course of the ocular disease. Among local and systemic treatments, no defined guidelines exist regarding which chemotherapy agent should be used either for intravitreal (IV) or systemic use, as well as when to choose radiotherapy of the orbit (10-12). Tumor recurrence within the eye is frequent, and the survival depends on CNS involvement. The reported mortality rate ranges between 9% and 81% in different follow-up periods, and the survival time is 12–35 months (13-15). The aim of this review is to summarize the most recent and useful tools, techniques and regimes for the diagnosis, management and treatment of patients with PVRL.
Classification and nomenclature
Intraocular lymphoma (IOL) is a rare malignancy that can affect every tissue within the eye with different morphologic, immunophenotypic, genetic and clinical features (1). IOL can be divided into primary IOL (PIOL), which is a particular subset of primary CNS lymphoma, and secondary IOL (SIOL) arising from a systemic lymphoma, outside the CNS. The most common form of PIOL by far is PVRL, that involves the vitreous, the retina, and the retinal pigment epithelium (RPE) (16). This condition can present as an isolated entity or develop before, after, or concurrently with brain lymphomatous involvement. Around 60–90% of PVRLs involve the brain subsequently, while 15–20% of patients with primary central nervous system lymphoma (PCNSL) will develop PVRL later (17). The majority of PVRL is of B-cell origin. However, T-cell lymphomas and T-cell rich lymphomas can also develop within the eye (18-20). SIOL most commonly involves the uvea, especially the choroid. Conversely, secondary involvement of the vitreous, retina, and RPE by systemic lymphoma is extremely rare and can mimic the features of PVRL (21). Whether the eye is the primary site of the lymphoma or becomes affected after CNS involvement, the intraocular findings are similar, as well as are similar the ophthalmic features of a primary or secondary vitreoretinal lymphoma.
PCNSL is a lymphoma that originates in the brain, spinal cord or leptomeninges, usually of diffuse large B-cells (DLBCL), non-Hodgkin, type. It accounts for 4–6% of primary brain tumors and 1–2% of extranodal lymphomas (22,23). Rarely PCNSL can be of T-cell origin.
Epidemiology
The incidence of PVRL is hard to be estimated because no central database exists for this disease. The approximate incidence is 0.047 cases per 100,000 people per year (24), representing 4–6% of all brain tumors and less than 1% of all non-Hodgkin’s lymphomas (25,26). The reasons for this increasing incidence are still unknown, rising from 0.027/100,000 in the seventies to 1/100,000 in the nineties. Hypotheses are the increased median age of the population and better diagnostic procedures (27). PCNSL/PVRL more commonly affect patients in the fifth-sixth decades of life. However, few cases of PVRL have been documented affecting childhood and adolescence, particularly in immunocompromised patients (28,29). There seems to be no predilection of sex or race. Few reports suggest women be more affected by PVRL than men, by 2:1 or more (30-32), while other studies suggest that men are more involved (32).
Etiology and pathogenesis
Despite the paucity of tissue specimens from PVRL, some insights into the biology and pathogenesis of PVRL can be extrapolated from the studies conducted in PCNSL (1). These studies demonstrated that PCNSL and PVRL manifest outside CNS very rarely, with the exception of the testis, highlighting the similarities between immunological sanctuaries (4). PCNSL and PVRL are associated with a worse prognosis than other localized extranodal subtypes of non-Hodgkin’s lymphomas, and they respond effectively to methotrexate (MTX). Indeed, progression-free survival (PFS) intervals are quite long with MTX-based monotherapy in approximately 20% of PCNSL patients (33).
Two different hypotheses exist about the development of PCNSL and PVRL. One hypothesis is that a malignant B-cell of systemic origin expresses selective molecules to migrate and home to CNS, where a second mutation facilitates the clonal growth. Another hypothesis is the infectious one, for which, in immunosuppressed patients, EBV infection of B-cells results in their immortalization and then the suppressed T-cell function leads the EBV-infected lymphocytes to evolve towards malignancy (34). Furthermore, Toxoplasma gondii infection was related to the development of B-cell lymphomas because its DNA was found in vitreous samples of PVRL patients (35).
PVRL cells are usually positive for CD20 and CD79a in immunostaining, both considered pan B-cell markers, and negative for CD3, a T cell marker. Typically, depending on the differentiation stage of the malignant B-cell, there are two groups of DLBCL, with different immunophenotypes and gene expression profiles: germinal center B-cell (GCB) type and activated B-cell (ABC) type. Considering the mutational pattern and the gene expression profile, PCNSL belong to ABC-type (4). Regarding PVRL, many discrepancies have been reported and consensus is lacking on considering it of ABC-type. The high frequency of t(14,18) chromosomal translocation, with the consequent high expression of Bcl-2 due to IgH-Bcl-2 rearrangement, suggested malignant cells in PVRL to originate from GCB cell (36). Moreover, gene expression suggests a pattern similar—but not identical—to GCB signature. On the other hand, Coupland et al. have determined the immunophenotype to be likely to ABC-type, with the positivity of MUM1/IRF4 and the loss of CD10 expression, according with a late germinal center differentiation stage origin. To note, however, that myeloid differentiation factor 88 (MYD88) and CD79B mutations are rare in GCB type but very frequent in PVRL (37).
MYD88 is a gene discovered in the 1990s as a primary differentiation response factor in myeloid precursors. It plays a key role in toll-like receptor signaling and it has been found mutated (L26P) at a high frequency in PVRL samples. Therefore, MYD88 L265P is considered a typical tract of PVRL mutational signature. In addition, a variable percentage of PVRL samples showed mutations in CD79b, involved in BCR signaling cascade, and other genes such as PIM1, IGLL5, BTG1 and CDKN2A (38). This mutational pattern suggested that Nf-kb pathway hyperactivation could play a pathogenetic role and put the basis to new therapeutic approaches. More than 90% of malignant cells test positive for BCL-2/IGH t(14;18) translocation. In addition, two genes group with different tendencies of gaining (BCL6, MYD88, MYC, CD79A, PTEN) and losing (CDKN2A, IGH, PTPRK, CD79B and BCL-2) copy number have been described (39).
In order to provide further insights on PVRL ontogenesis and its relationship with PCNSL, immunoglobulin genes have been sequenced. In PVRL samples, an overwhelming restricted immunoglobulin gene repertoire was highlighted, with a strong recurrence of IGHV4-34 gene (64%) (40). This consideration distinguishes PVRL from PCNSL, as a sub-group with its own peculiar characteristics. The particularly restricted immunoglobulins repertoire after somatic hypermutation suggests that antigen selection process is a major driver in lymphomagenesis and makes stronger the concept that PVRL malignant cells originate from a late differentiation stage in germinal center. Besides, IGHV4-34 antibodies were tested in large scale protein microarray and recognized several proteins, displaying a polyreactive behavior (41). However, the antigen driver has not yet been found.
Some chemokines involved in leukocyte trafficking, proliferation, and adhesion have been demonstrated to be ectopically expressed by retinal pigmented epithelium in PCNSL/PVRL. Some of them are CXCL13 and CXCL12. Chemokine receptors for CXCL13 and CXCL12—respectively CXCR5 and CXCR4—were found as well on malignant B-cells (42). The expression of these chemokines in immunologic sanctuaries, like the eye or the brain, contributes to the passage of lymphomatous cells from the choroidal circulation (i.e., systemic circulation) to the retina through RPE, crossing the blood-retinal barrier.
Other upregulated genes and more expressed molecules include c-myc, Pim-1, interleukin 4, and STAT-6 protein. Different studies have shown that molecular expression is different between different lymphoma subtypes. For example, osteopontin, chitinase or RGS-13 are more expressed in PCNSL, whereas collagen type IV, laminin α-4, and lumican are expressed at higher levels in systemic lymphomas (43).
Also, significantly higher expression of the microRNA miR-17-5p has been demonstrated in PCNSL rather than in nodal and testicular DLBCL (44). Unfortunately, the mechanism involved in the migration of tumor cells between the eye and the brain that leads to the development of PVRL from PCNSL, and vice versa, remains still unknown. Minezaki et al. carried out a mi-RNA profiling in VRL both in vitreous and in serum samples: different mi-RNAs have been found to be down or upregulated, mainly involving tumorigenesis pathways. At the same time, miR-361-3p has been proposed as a possible novel diagnostic tool, for discriminating VRL from others uveitis (45).
In conclusion, PVRL has been proposed to be an independent sub-type of DLBCL, with peculiar characteristics and gene expression pattern.
Common clinical manifestations
PVRL has been defined a “masquerade syndrome” as it can mimic a variety of ocular diseases, usually intermediate and posterior uveitis. Moreover, clinical manifestations often vary between patients. PVRL is bilateral in 64–83% of the cases and generally asymmetrical (1,21).
The majority of patients complain about hazy vision and floaters occurring for several months. Only a few refer to the ophthalmologist for severe decreased visual acuity. Patients experience improvement of symptoms after corticosteroid therapies, either oral or local, that are often prescribed by the clinician who had misdiagnosed PVRL for uveitis. Mild, nonspecific ocular symptoms and the benefit to corticosteroid therapy explain in part the diagnostic delay; from symptoms onset, there is a diagnostic delay between 6 months to 2 years to reach the definite diagnosis (1,46).
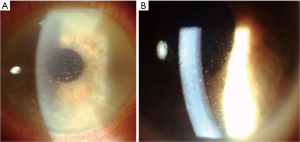
Anterior segment manifestations are present in about 50% of PVRL patients and comprehend granulomatous or non-granulomatous keratic precipitates (Figure 1), few cells and mild flare in the anterior chamber. More rarely, pseudohypopyon and iris/angle infiltration have been described.
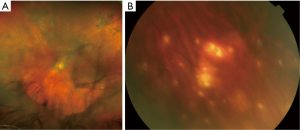
Intermediate and posterior manifestations include mild to severe vitritis characterized by cells that organize in clumps, strands, and sheets along vitreous fibrils (Figure 2A). Cells are larger than ordinary inflammatory cells and do not cluster with reactive cells. Retinal manifestations are heterogeneous and may be non-specific. Creamy yellowish infiltrates are the most common features and can be located at the posterior pole or in the peripheral retina (Figure 2B). The most common fundal appearance consists of pin points yellowish lesions named “leopard spots”. Infiltrates can be small, focally located or diffuse, or can be large lesions occupying the entire fundus in advanced stages. In a small number of cases, serpiginous-like or retinitis-like lesions have been reported (17,47). Non-specific manifestations include vasculitis, exudative retinal detachment, RPE atrophy with subretinal fibrosis, disciform scarring at the macula, and optic nerve edema (2,48).
PVRL of T-cell origin presents, overall, with more severe anterior segment inflammation and keratic precipitates usually of granulomatous type, cells and flare in the anterior chamber. Other manifestations include vitritis, inflammatory glaucoma, macular edema and choroidal detachment (49). A myriad of neurological symptoms can develop during the course of the disease due to CNS involvement. They include behavioral changes, alteration of cognitive functions, hemiparesis, ataxia, seizures, and others (50).
Imaging
Multimodal imaging has been considered one of the uttermost recent revolutions in the ophthalmic field. PVRL presents some typical imaging characteristics. However, being a masquerade syndrome, it can resemble other types of posterior segment diseases.
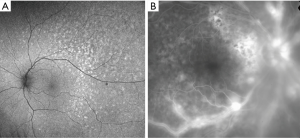
In the majority of cases, fundus autofluorescence (FAF) shows hypo- and hyperautofluorescent areas corresponding to RPE atrophy and lymphomatous infiltration, respectively (Figure 3A). FA has been reported to show several findings, including punctate hyperfluorescent window defects and hypofluorescent lesions (“leopard spot” appearance). On FA, hypofluorescent spots may represent retinal lymphomatous infiltrates which give blockage to the dye, whereas hyperfluorescent spots represent atrophic lesions with window defects through RPE. Other fluorescein findings are perivascular leakage (Figure 3B), papillary leakage, and more rarely macular breakdown of blood-retinal barrier in the form of macular edema. Indocyanine green angiography (ICGA) often shows round clustered hypocianescent lesions corresponding to the areas of hypofluorescence on FA, but it may be completely normal or non-contributory. Other angiographic features are represented by granularity, blockage, and late staining at the level of RPE. OCT is of great aid in the diagnosis of PVRL. In addition, it is of paramount importance in the follow-up to compare retinal findings without the need of an invasive technique and the use of a dye. Focal infiltrations of lymphomatous cells can be found under the RPE-Bruch’s membrane complex or in the subretinal space (Figure 4). Infiltrations appearance can vary, going from subtle RPE hyperreflective mottling to focal discrete hyperreflective nodularity under RPE or under the retina, to large hyperreflective lesions that create confluent bands of material under the retina, or solid RPE detachments. RPE detachments can be initially isolated and discrete, but then, with proliferation and spread of the disease, they can enlarge developing wide sheets of hyperreflective material below RPE and below the retina. Sub-RPE infiltration has been proposed as a possible marker of intra-ocular tumor recurrence and poorer visual acuity outcome (51). Conversely, there is not a univocal association between sub-RPE infiltration and CNS involvement or overall survival (OS). Dalvin et al. found that sub-RPE infiltrates were related to a lower survival time, compared to their absence (46 and 76 months, respectively) (52).
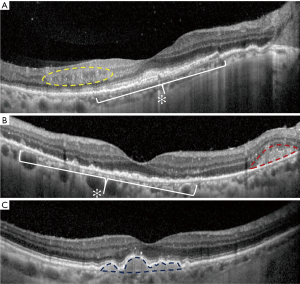
Other OCT findings include disruption of the photoreceptor ellipsoid zone, exudative retinal detachments with hyporeflective fluid, multiple band of hyperreflective material within the inner retina, and hyperreflective foci in the posterior vitreous. Moreover, vertical hyperreflective lesions extending from the outer to the inner retina have also been described and interpreted as retinal infiltrations (53). Recently, PVRL has been described with features resembling an infectious retinitis. At fundus examination, retinitis-like lesions appear as yellow-white patches with few retinal hemorrhages and mild vascular occlusion. OCT imaging shows either full-thickness retinitis-like lesions or partial-thickness retinitis from the inner limiting membrane to the outer nuclear layer.
The distinguishing features of PVRL retinitis-like lesions, compared to infectious retinitis (caused by herpes viruses and toxoplasma), are the presence of homogeneous hyperreflective infiltrates in the sub-RPE space and a “rounded roof” appearance. Also, retinal thickness of retinitis-like lesions in PVRL patients is significantly higher compared to infectious retinitis. Conversely, viral retinitis is significantly more associated with anterior uveitis, iris atrophy and fundal lesions with sharp, well-defined edges; whereas, toxoplasma lesions are unifocal, had less retinal hemorrhages and more chorioretinal scars (47).
Ultra-widefield imaging (pseudocolor fundus photography, FA, ICGA, and FAF) is an adjunctive tool that aids the clinician in obtaining a panoramic view of the disease features. With pseudocolor wide-field imaging, vitreous can appear characterized by uniform material with non-specific features, by the “string of pearls” pattern (round yellowish clusters of cells organized in lines) and the recently described “aurora borealis” pattern. The aurora borealis pattern was defined as linear opacities and sheets with a myriad of cells uniformly aligned along vitreous fibrils, scattering the light beam, resembling the so-named natural phenomena. Vitreous liquefaction status and syneresis may explain different patterns: a more liquid vitreous usually shows unspecific patterns, while a preserved structure may act as a scaffold for linear disposition of lymphomatous cells in fibrils (aurora borealis or string of pearls) (54) (see Table 1).
Table 1
| Presence of malignant B-cell lymphocytes in vitreous and retinal samples |
| Scanty basophilic cytoplasm |
| Increased nucleus: cytoplasm ratio |
| Hypersegmented nuclei with various shape and number |
| Coarse chromatin pattern |
| Immunohistochemistry in vitreous and retinal samples |
| CD19+ |
| CD20+ |
| CD22+ |
| k/λ ratio >3 or <0.6 |
| High IL10/IL6 ratio in aqueous and vitreous samples |
| Novel gene mutations |
| MYD88L265P in aqueous and vitreous samples |
| CD79B in vitreous samples |
| Other interleukin expression in vitreous samples (IL35) |
B-scan ultrasound is useful to check retinal integrity when the media are opaque, as well as elevated chorioretinal lesions, and optic nerve widening (54).
Magnetic resonance imaging (MRI) with gadolinium-based contrast of the brain is imperative for every patient with PVRL, both at the time of the diagnosis and on a regular basis during the follow-up. MRI helps in identifying CNS involvement as well as checking the response of the brain lesions to the treatment. Neurological lesions appear hypodense on T1-weighted and hyperdense on T2-weighted scans with discrete or diffuse borders (55).
Positron emission tomography/computed tomography (PET/CT) of the brain aids in identifying the activity of both PCNSL and PVRL. PCNSL has an uptake value 2.5 times higher than gray matter and its uptake pattern helps in the differential diagnosis of others intracranial tumors. Moreover, early changes in 18F-FDG uptake after 3 weeks of chemotherapy has prognostic value, having higher sensitivity than conventional MRI (56,57). Furthermore, whole body PET/CT investigate a possible extra-CNS malignancy with CNS dissemination.
Diagnosis
PVRL diagnosis cannot be defined without clinical ocular examination and imaging techniques. However, under clinical suspect, the gold standard for definite PVRL diagnosis remains histopathologic examination of the ocular specimens, with demonstration of malignant B lymphocytes in the vitreous or retina, and immunohistochemistry to characterize lymphocyte type and clonality (10,58). Vitreous biopsy is the procedure that is most frequently performed in clinical setting. Conversely, chorioretinal biopsy is confined to challenging or doubtful cases, for example when the vitreous biopsy has not been detrimental and the disease is progressing despite the treatment. Trans pars plana vitrectomy with 25 or 27 G instrumentation is the gold standard. Low vitrector cut rate (600 cuts per minute or less) under air infusion is the preferable technique for diagnostic vitrectomy, to avoid cell damage and globe hypotony. Undiluted sample is collected for cytopathological evaluation (59-61). Apart from the diagnostic purpose, vitrectomy aims at cleaning the vitreous from debris in order to improve visual acuity, becoming a therapeutic procedure, as well. Samples should be transferred immediately to the laboratory for the analysis without fixation, to preserve morphology and immunoreactivity. Alternatively, vitreous samples could be fixed in mild fixative-agents, like herpes/glutamic acid buffer-mediated organic solvent protection effect (HOPE) fixation or Cytolyt (Cytyc) for subsequent ThinPrep slide preparation. Formalin fixation should be avoided, because it damages cell morphology and immunoreactivity (62). Overall, diagnosis is challenging due to several factors, including the limited material of the vitreous biopsy and its fragile nature whose management requires particular attention, the low number of neoplastic lymphocytes, the previous treatment with corticosteroids and the skill and experience of the cytopathologist. All these factors result in a high rate of false-negative vitreous biopsies (2). Morphologically, malignant lymphomatous cells are characterized by scanty basophilic cytoplasm, increased nucleus: cytoplasm ratio, hypersegmented nuclei of various shape and number, and a coarse chromatin pattern. The positive predictive value of cytologic evaluation alone is around 31%, and the negative predictive value is approximately 33%, due to the sparse number of cells, necrotic debris, and “contaminating cells” like reactive T-lymphocytes. A particular PVRL-cytokine profile has been reported both in the aqueous and in the vitreous, with a remarkable elevation of IL-10. IL-10, indeed, is an interleukin produced by B-cells and, when found at high levels, it is linked to rapid disease progression (38,63-65). However, there is not a defined and commonly accepted threshold for IL-10 levels. IL-10/IL-6 ratio is considered more informative. IL-6 is a cytokine expressed during inflammatory processes like uveitis that may help to distinguish a pure inflammatory process from a masquerade syndrome, like lymphoma. When an elevated IL-10/IL-6 ratio is detected, the positive predictive value for PVRL is 95% and the negative predictive value is 71% (10-12). Nevertheless, a low IL-10/IL-6 ratio does not automatically exclude IOL. Costopoulos et al. showed a limited sensibility of the IL-10/IL-6 ratio. Thus, they developed and proposed a new score—the Interleukin Score for intra-Ocular Lymphoma Diagnosis (ISOLD)—valid for both aqueous and vitreous samples. This score is given from IL10 and IL6 concentrations, and its value could predict the probability of having PVRL. For aqueous humor, the ISOLD formula is: −12.871+5.533× log(IL-10 +1) −1.614× log(IL-6 +1). For vitreous, the ISOLD formula is: −12.208+4.648× log(IL-10 +1) −1.669× log(IL-6 +1). When the ISOLD score value is <−4.6, they reported a >99% probability for a patient not to have lymphoma. Conversely, a value >+4.6 was strongly indicative for it. In that study, only 6% of PVRL patients totalized a score in the “grey zone”, ranging from −4.6 to +4.6 with a less strong diagnostic capability (66). ISOLD score has already been validated and it is a valid tool for the differential diagnosis between lymphoma and uveitis (67).
Takeda et al. studied different interleukin expression in vitreous: IL-10, IL-20, IL-22, IL-27, IL-35 and soluble IL-22 receptor α were significantly different in VRL patients compared to those patients with different non-infective uveitis. Moreover, high level of IL-35 was associated with poor outcome, highlighting a putative prognostic role (68). Cells phenotyping by their surface markers is conducted by immunocytological techniques and flow cytometry, which use respectively antibodies directed to a specific marker, and a cell sorter that separates cells in a fluid medium. Malignant B-cells stain positively for CD19, CD20, and CD22 with restricted expression of either k or λ chain. A ratio of k:λ >3 or <0.6 is indicative of monoclonality, as the normal ratio in inflammatory conditions, like uveitis, is around 1 (38,69). Conversely, T-cell population stains positively for CD3 and CD4. Reasons for false-negative results include B-cell lymphomas that are too poorly differentiated to express CD20 or light chains on the cell surface, or large numbers of reactive T-cells that may obscure the malignant B-cell component. In this latter case, the diagnosis is often delayed because the B-cell phenotype is masked by reactive inflammation (18). PCR (polymerase chain reaction) amplification can be used if malignant cells are too scanty in vitreous specimens. It has been used to detect gene rearrangements of the complementarity defining sequences in the variable region of the heavy chain of B-cells (CDR3) and translocations of bcl-2 proto-oncogene. For T-cell lymphomas, the primers target the T-cell receptor gamma (TCR). Recently, MYD88 L265P mutation has been found with a high prevalence in DLBCL, and in vitreous specimens from PVRL (70). In addition, Yonese et al. reported CD79B mutation in 35% of PVRL vitreous samples. CD79B encodes the Ig-β, a structural protein in B-cell receptor which plays a key role in BCR signaling (71). Aqueous tap, as well, has been considered a viable diagnostic procedure for the detection of MYD88 L265P mutation. Being a simple and safer procedure compared to diagnostic vitrectomy, aqueous tap has been proposed as an adjunctive and complementary diagnostic strategy for early detection of PVRL. Moreover, detecting a gene that can be found even in the absence of intact cells overcomes all the drawbacks of the cytology analysis.
Aqueous sampling can be performed either in the operating theatre or just under direct visualization at the slit lamp. After topic anesthesia with drops, a 29-gauge insulin syringe is inserted at the peripheral cornea at the temporal side, and approximately 0.3 mL of aqueous humor is collected. MYD88 L265P mutation analysis is performed extracting DNA from the aqueous sample using the Qiamp DNA Mini-Kit (Qiagen Gmbh, Hilden, Germany). The codon 265 mutation assay is performed using the amplification-refractory mutation system polymerase chain reaction approach. The further pyrosequencing analysis is done by using PyroMark Gold Q96 (Qiagen) reagents with PyroMark Q96ID. It is noteworthy that a strong concordance of positive results between vitreous samples and aqueous samples from the same eye was demonstrated.
The main limit in performing multiple analyses on the same sample is represented by its small volume. However, a recent technique, the metagenomic deep sequencing, bypasses this limit and allows to detect mutations using a very small volume (20–50 µL) of fluid with an unbiased approach. Its use may help not only for PVRL diagnosis, but could provide prognostic elements like mutations that confer chemo-resistance or chemo-sensitivity (70,72). Moreover, MYD88 mutation has been supposed as a viable tool during therapy for disease monitoring, since mutation negativization accompanies clinical improvement (73) (see Table 2).
Table 2
| Fundoscopy/color fundus photography |
| Vitreitis (vitreous sheets, aurora borealis pattern, string of pearls pattern) |
| Yellowish retinal lesions of different shapes and size |
| Optical coherence tomography |
| Sub-retinal pigment epithelium infiltrations |
| Subretinal infiltrations |
| Vertical hyperreflective lesions in the retina |
| Retinitis-like appearance (full thickness or partial thickness) |
| Autofluorescence |
| Punctate hyperautofluorescent and hypoautofluorescent lesions |
| Fluorescein angiography |
| Punctate hyperfluorescent and hypofluorescent lesions (leopard spot appearance) |
| Perivascular leakage |
| Papillary leakage |
| Indocyanine green angiography |
| Round clustered hypocianescent lesions |
Cerebrospinal fluid (CSF) evaluation should be performed in every case of PCNSL suspect. Around 25% of patients with identifiable lesions on MRI will have positive CSF cytology (74). Extensive blood examinations should be performed to rule out other causes of infectious and non-infectious uveitis. A complete blood count, HIV and EBV serology are useful tests to understand the systemic clinical status of the patient.
Treatment
Several local and systemic therapies are available as treatments for PVRL, but the optimal therapy has not been defined. The crucial point in defining the therapeutic decision is CNS involvement. Thus, PVRL diagnosis must be followed by gadolinium-based MRI of the brain. Treatment goals in PVRL without PCNSL are both the control of intraocular disease and the prevention of CNS dissemination, which occurs in 60–90% of patients. It is still debated to give or not systemic treatment to patients with isolated PVRL, and predictive factors for CNS dissemination have not definitely been found (4). De la Fuente et al. treated PVRL patients with bilateral radiation therapy followed by systemic MTX and recorded that the incidence of CNS spread (37.5% with a median of 68 months follow-up) (46) was lower than reported from other studies (from 56% to 85%) (1,75). The rationale of this management is that several PVRL patients could have undetectable occult CNS involvement that cannot be managed with local therapies only, but they require systemic chemotherapy to avoid macroscopic CNS dissemination (45). Baron et al. gave temozolomide monotherapy in relapsing or refractory disease or not eligible to high-dose methotrexate (HD-MTX) patients, showing low toxicity and good overall response (76).
Other studies have shown a remarkable benefit combining IV and systemic MTX in the PFS, but not in the OS. On the other hand, Riemens et al. did not show superiority of this combined strategy versus local treatment alone (10). A study by Hormigo et al. showed a significantly longer median survival rate in PVRL treated with prophylactic chemotherapy and/or radiotherapy compared to the group treated after CNS signs had developed (5).
There is a univocal consensus about the need of novel instruments, both clinical and non-clinical, to stratify the risk of CNS involvement in PVRL patients. The main goal is to identify that group of patients who would receive a benefit from systemic therapy. The International Primary CNS Lymphoma Collaborative Group suggested high doses of systemic chemotherapy along with IV chemotherapy and/or ocular radiotherapy, even in the absence of PCNSL, in 2011 (1). A study by Hashida et al. demonstrated that prophylactic systemic chemotherapy, despite not inhibiting, significantly delays the onset of CNS disease (77). However, there is still no consensus about treating isolated PVRL with systemic chemotherapy to prevent CNS involvement.
Local therapies include radiotherapy and IV chemotherapy. There are no that compare these two treatment options, and whether to use one or the other as first-line therapy. The choice should be made based on disease laterality, patient preference, and other practical considerations. External-beam radiotherapy (EBRT) has been recommended for patients with PVRL without CNS involvement. It typically consists of a total of 35–40 Gy delivered in approximately 15 fractions of 2 Gy each, from opposed lateral beams to include both eyes. With this dosage, recurrence and radiation retinopathy rates have been reported to be very low. Cataract formation, that is a common complication, can be easily managed surgically. Whole-brain plus eye radiotherapy can be added if a patient with CNS involvement had failed systemic chemotherapy or is debilitated to undergo aggressive therapies. However, complications such as decreased cognitive functions, ataxia, or even death should be taken into consideration (78).
IV treatment consists of a delivery of a drug into the eye by means of an injection. In PVRL, variable doses and regimens of MTX and/or rituximab injections have been proposed. The most common therapeutic scheme consists of IV MTX administered at a dose of 400 µg in 0.1 mL twice a week for four weeks—induction phase, then once a week for eight weeks—consolidation phase, then once a month for nine months—maintenance phase, for a total of twenty-five injections. With this treatment scheme, recurrences have been shown to be very rare and only a few complications have been described, like corneal epitheliopathy and transient rise in intraocular pressure (79). Alternatively, IV rituximab is administered at the dose of 1 mg/0.1 mL for four weeks, repeating the course based on clinical response. Zhou et al. proposed a reduced frequency IV MTX injections, switching directly from induction to maintenance phase. They observed a lower risk of corneal epitheliopathy without changes in therapeutic effects (80). Giuffrè et al. used a combined IV treatment alternating MTX and rituximab for 4 weeks, and then every two weeks for 3 months, with good clinical outcomes (17). In a study by Cicinelli et al., 44% of patients showed a complete disappearance of PVRL, while 56% displayed partial or no remission after three injections of rituximab (81). New biological agents like PDL-1 inhibitors, nivolumab and BTK-inhibitors are under study as treatments for PCNSL (79).
Regarding systemic therapy, there are two stages of treatment: induction and consolidation. Induction treatment includes HD-MTX (alone or combined) (82-84). Several studies have demonstrated an optimal response to MTX in PVRL with CNS or systemic involvement. Rates of remission up to 72% and up to 94–100% have been shown when used alone or in combination with other therapies, respectively. After the International Extranodal Lymphoma Study Group (IELSG) 32 trial, the use of MATRix combination (MTX, cytarabine, thiotepa and rituximab) has been defined as new standard chemotherapy for patients <70 years as first-line treatment for PCNSL. The complete remission rate at 30 months in the group treated with MATRix regimen was around 50% compared to only 23% in the group treated with MTX and cytarabine, and 30% in the group treated with MTX, cytarabine and rituximab (85). In elderly patients, HD-MTX was combined with different cytotoxic agents leading to better results than HD-MTX alone. However, due to the low number of data, there is no evidence that a specific combination regimen is better than the others (86). Whole brain radiation therapy (WBRT) could be combined to MATRix protocol, but the risk of cytotoxicity and a poor quality of life must be considered (87). Thus, WBRT is an option in rescue or palliative treatment. In addition, intrathecal therapy—often rituximab—should be considered in those patients with a poor response to HD-MTX or not fit to receive a minimum dose of MTX 3 g/m2 (88).
After HD-MTX induction therapy, 60% of patients usually achieve complete response. Given the risk of disease relapse, they still need consolidation therapy. Consolidation therapy includes WBRT, additional chemotherapy or high-dose chemotherapy followed by autologous stem cell transplantation (ASCT). IESLG-32 trial showed no significant differences in OS between WBRT and thiotepa-based myeloablative therapy followed by ASCT, but highlighted different side effects namely neurotoxicity and myelotoxicity, respectively (88).
In case of relapsing disease, there are different treatment options. HD-MTX could be repeated if there was a good response during the induction therapy. Other options include thiotepa-based chemotherapy followed by ASCT, intrathecal cytarabine, high dose cytarabine and pemetrexed, lenalidomide, pomalidomide or ibrutinib.
Ibrutinib has been investigated as a possible option in relapsing and refractory PCNSL and PVRL as inhibits BTK, a kinase involved in BCR signaling, a proliferative driver in lymphomas. Ibrutinib showed therapeutic benefit, but a clear correlation between mutational profile and ibrutinib sensitivity has not been established (89). The presence of MYD88 L265P mutation has been demonstrated to confer a potential sensitivity to ibrutinib in Waldenstrom macroglobulinemia, and CD79b mutation seems to confer drug resistance (90) (see Table 3).
Table 3
| Local treatment |
| External-beam radiotherapy (35–40 Gy in 15 fractions of 2 Gy each) |
| Intravitreal (IV) treatment: |
| IV methotrexate (400 μg/0.1 mL) |
| Induction: twice a week for four weeks |
| Consolidation: once a week for eight weeks |
| Maintenance: once a month for nine months |
| IV rituximab (1 mg/0.1 mL) |
| Once a week for four weeks |
| Repeat the course based on clinical response |
| Alternating IV methotrexate and rituximab: |
| Induction: once a week for 4 weeks |
| Consolidation: every two weeks for 3 months |
| Systemic treatment |
| Induction therapy: |
High dose methotrexate-based (HD-MTX) therapy (MATRix if patient ≤70 years)
Consider rituximab intrathecal therapy in case of poor response to HD-MTX or if MTX dose is less than 3 g/m2
Consolidation therapy:
Whole brain radiation therapy; or
HD chemotherapy followed by autologous stem cell transplantation
Relapsed or recurrent disease:
Thiotepa-based chemotherapy followed by ASCT or
Intrathecal cytarabine or
High dose cytarabine and pemetrexed, lenalidomide, pomalidomide or ibrutinib
PCNSL, primary central nervous system lymphoma.
Prognosis
The presence of sub-RPE infiltrates has been proposed as a negative prognostic factor in PVRL for OS, PFS and visual outcome, but the data did not reach statistical significance (51). At the same time IL-35 vitreous level seems to play a prognostic role in VRL, but again, a small number of patients has been considered in the study (68). The IELSG score is the most important prognostic score in PCNSL; therefore, it is limited to PVRL with cerebral involvement. This score considers LDH, CSF proteins, age greater than 60 years, lymphoma location (basal ganglia, periventricular zone, brainstem, cerebellum) and Eastern Cooperative Oncology Group performance status (84,91).
PVRL is a masquerade syndrome and its diagnosis is often delayed. This may partially explain the high rate of CNS involvement and the low survival rate in long-time follow-up. PVRL with associated PCNSL has poor ocular and “quoad vitam” prognosis for the intrinsic aggressive nature of the disease. The rate of mortality is difficult to attest existing variable reports in the literature due to different patient populations, treatment regimens, and follow-up. Mortality ranges from 9% to 81%, and median survival time goes from 12 to 35 months in different studies (13,20,30,92).
Conclusions
Vitreoretinal lymphoma is the most common type of ocular masquerade syndrome. Its diagnosis and treatment are challenging and no guidelines exist. It is crucial to send patients with PVRL suspect to tertiary referral hospital that manage a conspicuous number of patients affected by this malignancy. It is equally important to collect as much data as possible from these tertiary referral hospitals, to share knowledge and improve our understanding of the disease. Clinical suspicion is essential in case of posterior uveitis with suggestive lymphoma findings and temporary response to corticosteroids. It is advisable nowadays to perform a complete multimodal imaging with widefield color fundus photography, autofluorescence, FA, ICGA and OCT to rule out other inflammatory conditions and support the suspect of vitreoretinal lymphoma. A high level of expertise is required when analyzing these pictures as a misleading diagnosis could delay proper treatment with negative implications on visual outcome and disease progression.
Despite cytology and immunohistopathology of vitreous and retinal specimens is the gold standard for the diagnosis, it requires an invasive procedure with high rates of false negatives. We recommend to perform always bilateral aqueous tap, which is an easy to perform technique, in order to detect MYD88 265P and then proceed with the more invasive vitrectomy procedure.
The best therapeutic option for vitreoretinal lymphoma has not yet been defined. While several studies exist regarding PCNSL therapeutic approach with a clear efficacy of HD-MTX based systemic therapy, just few data are available on vitreoretinal lymphoma without CNS involvement. We recommend to treat isolated vitreoretinal lymphoma with orbital radiotherapy or IV injections depending on the general conditions of the patient, requiring the injections more visits to the hospital. Unluckily, it is not yet clear if systemic therapy given to patients with only intraocular malignancy could delay or prevent the onset of CNS disease.
Finally, prognostic factors and predictive features for CNS involvement should be identified. That could allow a proper risk stratification and a better management of the disease, improving patients’ life quality and survival.
A desperate need for shared information and definite guidelines exists among ophthalmologists and hematologists. We are aware that future large clinical trials are required to assess the best management of this potentially fatal disease.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Andrés J. M. Ferreri, Maurilio Ponzoni) for the series “Central Nervous System Lymphomas” published in Annals of Lymphoma. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aol-20-27). The series “Central Nervous System Lymphomas” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chan CC, Rubenstein JL, Coupland SE, et al. Primary vitreoretinal lymphoma: a report from an International Primary Central Nervous System Lymphoma Collaborative Group symposium. Oncologist 2011;16:1589-99. [Crossref] [PubMed]
- Davis JL. Diagnosis of intraocular lymphoma. Ocul Immunol Inflamm 2004;12:7-16. [Crossref] [PubMed]
- Sen HN, Bodaghi B, Le Hoang P, et al. Primary Intraocular Lymphoma: Diagnosis and Differential Diagnosis. Ocul Immunol Inflamm 2009;17:133-41. [Crossref] [PubMed]
- Fend F, Ferreri AJM, Coupland SE. How we diagnose and treat vitreoretinal lymphoma. Br J Haematol 2016;173:680-92. [Crossref] [PubMed]
- Hormigo A, Abrey L, Heinemann MH, et al. Ocular presentation of primary central nervous system lymphoma: diagnosis and treatment. Br J Haematol 2004;126:202-8. [Crossref] [PubMed]
- Farrall AL, Smith JR. Eye involvement in primary central nervous system lymphoma. Surv Ophthalmol 2020;65:548-61. [Crossref] [PubMed]
- Mochizuki M, Singh AD. Epidemiology and clinical features of intraocular lymphoma. Ocul Immunol Inflamm 2009;17:69-72. [Crossref] [PubMed]
- Gonzales JA, Chan CC. Biopsy techniques and yields in diagnosing primary intraocular lymphoma. Int Ophthalmol 2007;27:241-50. [Crossref] [PubMed]
- Miserocchi E, Ferreri AJM, Giuffrè C, et al. MYD88 L265P mutation detection in the aqueous humor of patients with vitreoretinal lymphoma. Retina 2019;39:679-84. [Crossref] [PubMed]
- Riemens A, Bromberg J, Touitou V, et al. Treatment strategies in primary vitreoretinal lymphoma: a 17-center European collaborative study. JAMA Ophthalmol 2015;133:191-7. [Crossref] [PubMed]
- Grimm SA, McCannel CA, Omuro AM, et al. Primary CNS lymphoma with intraocular involvement: International PCNSL Collaborative Group report. Neurology 2008;71:1355-60. [Crossref] [PubMed]
- Giuliari GP, Hinkle DM, Foster CS. Local treatment for lymphoid malignancies of the eye. Anticancer Agents Med Chem 2009;9:1123-8. [Crossref] [PubMed]
- Akpek EK, Ahmed I, Hochberg FH, et al. Intraocular-central nervous system lymphoma: clinical features, diagnosis, and outcomes. Ophthalmology 1999;106:1805-10. [Crossref] [PubMed]
- Dunleavy K, Wilson WH. Primary intraocular lymphoma: current and future perspectives. Leuk Lymphoma 2006;47:1726-7. [Crossref] [PubMed]
- Isobe K, Ejima Y, Tokumaru S, et al. Treatment of primary intraocular lymphoma with radiation therapy: a multi-institutional survey in Japan. Leuk Lymphoma 2006;47:1800-5. [Crossref] [PubMed]
- White VA. Understanding and Classification of Ocular Lymphomas. Ocul Oncol Pathol 2019;5:379-86. [Crossref] [PubMed]
- Giuffrè C, Cicinelli MV, Marchese A, et al. Clinical experience in a large cohort of patients with vitreoretinal lymphoma in a single center. Ocul Immunol Inflamm 2020; Epub ahead of print. [Crossref] [PubMed]
- Cummings TJ, Stenzel TT, Klintworth G, et al. Primary intraocular T-cell-rich large B-cell lymphoma. Arch Pathol Lab Med 2005;129:1050-3. [PubMed]
- Cao X, Shen D, Callanan DG, et al. Diagnosis of systemic metastatic retinal lymphoma. Acta Ophthalmol 2011;89:e149-e154. [Crossref] [PubMed]
- Coupland SE, Anastassiou G, Bornfeld N, et al. Primary intraocular lymphoma of T-cell type: Report of a case and review of the literature. Graefes Arch Clin Exp Ophthalmol 2005;243:189-97. [Crossref] [PubMed]
- Coupland SE, Damato B. Understanding intraocular lymphomas. Clin Exp Ophthalmol 2008;36:564-78. [Crossref] [PubMed]
- Hochberg FH, Miller DC. Primary central nervous system lymphoma. J Neurosurg 1988;68:835-53. [Crossref] [PubMed]
- Paulus W. Classification, pathogenesis, and molecular pathology of primary CNS lymphoma. J Neurooncol 1999;43:203-8. [Crossref] [PubMed]
- Levasseur SD, Wittenberg LA, White VA. Vitreoretinal lymphoma: a 20-year review of incidence, clinical and cytologic features, treatment, and outcomes. JAMA Ophthalmol 2013;131:50-5. [Crossref] [PubMed]
- Sagoo MS, Mehta H, Swampillai AJ, et al. Primary intraocular lymphoma. Surv Ophthalmol 2014;59:503-16. [Crossref] [PubMed]
- Meunier J, Lumbroso-Le Rouic L, Vincent-Salomon A, et al. Ophthalmologic and intraocular non-Hodgkin’s lymphoma: a large single centre study of initial characteristics, natural history, and prognostic factors. Hematol Oncol 2004;22:143-58. [Crossref] [PubMed]
- Schabet M. Epidemiology of primary CNS lymphoma. J Neurooncol 1999;43:199-201. [Crossref] [PubMed]
- Wender A, Adar A, Maor E, et al. Primary B-cell lymphoma of the eyes and brain in a 3-year-old boy. Arch Ophthalmol 1994;112:450-1. [Crossref] [PubMed]
- Sobrin L, Dubovy SR, Davis JL, et al. Isolated, bilateral intraocular lymphomain a 15 year-old girl. Retina 2005;25:370-3. [Crossref] [PubMed]
- Berenbom A, Davila RM, Lin HS, et al. Treatment Outcomes for Primary Intraocular Lymphoma: Implications for External Beam Radiotherapy. Eye (Lond) 2007;21:1198-201. [Crossref] [PubMed]
- Venkatesh R, Bavaharan B, Mahendradas P, et al. Primary vitreoretinal lymphoma: prevalence, impact, and management challenges Clin Ophthalmol 2019;13:353-64. [Crossref] [PubMed]
- Qualman SJ, Mendelsohn G, Mann RB, et al. Intraocular lymphomas. Natural history based on a clinicopathologic study of eight cases and review of the literature. Cancer 1983;52:878-86. [Crossref] [PubMed]
- Batchelor T, Carson K, O’Neill A, et al. Treatment of primary CNS lymphoma with methotrexate and deferred radiotherapy: A report of NABTT 96- 07. J Clin Oncol 2003;21:1044-9. [Crossref] [PubMed]
- Thomas JA, Crawford DH, Burke M. Clinicopathologic implications of Epstein-Barr virus related B cell lymphoma in immunocompromised patients. J Clin Pathol 1995;48:287-90. [Crossref] [PubMed]
- Shen DF, Herbort CP, Tuaillon N, et al. Detection of Toxoplasma gondii DNA in primary intraocular B-cell lymphoma. Mod Pathol 2001;14:995-9. [Crossref] [PubMed]
- Wallace DJ, Shen D, Reed GF, et al. Detection of the bcl-2 t(14;18) translocation and proto-oncogene expression in primary intraocu- lar lymphoma. Invest Ophthalmol Vis Sci 2006;47:2750-6. [Crossref] [PubMed]
- Coupland SE, Heimann H, Bechrakis NE. Primary intraocular lymphoma: a review of the clinical, histopathological and molecular biological features. Graefes Arch Clin Exp Ophthalmol 2004;242:901-13. [Crossref] [PubMed]
- Lee J, Kim B, Lee H, et al. Whole exome sequencing identifies mutational signatures of vitreoretinal lymphoma. Haematologica 2020;105:e458-60. [Crossref] [PubMed]
- Tan WJ, Meng MW, Ricciardi-Castagnoli P, et al. Single B-cell Genomic Analyses Differentiates Vitreoretinal Lymphoma from Chronic Inflammation. Ophthalmology 2020; Epub ahead of print. [Crossref] [PubMed]
- Belhouachi N, Xochelli A, Boudjoghra M, et al. Primary Vitreoretinal Lymphomas Display a Remarkably Restricted Immunoglobulin Gene Repertoire. Blood Adv 2020;4:1357-66. [Crossref] [PubMed]
- Montesinos-Rongen M, Purschke FG, Brunn A, et al. Primary Central Nervous System (CNS) Lymphoma B Cell Receptors Recognize CNS Proteins. J Immunol 2015;195:1312-9. [Crossref] [PubMed]
- Chan CC, Shen DF, Hackett JJ, et al. Expression of Chemokine Receptors, CXCR4 and CXCR5, and Chemokines, BLC and SDF-1, in the Eyes of Patients With Primary Intraocular Lymphoma. Ophthalmology 2003;110:421-6. [Crossref] [PubMed]
- Khan RB, Shi W, Thaler HT, et al. Is intrathecal methotrexate necessary in the treatment of primary CNS lymphoma? J Neurooncol 2002;58:175-8. [Crossref] [PubMed]
- Cloonan N, Brown MK, Steptoe AL, et al. The miR-17-5p microRNA is a key regulator of the G1/S phase cell cycle transition. Genome Biol 2008;9:R127. [Crossref] [PubMed]
- Minezaki T, Usui Y, Asakage M, et al. High-Throughput MicroRNA Profiling of Vitreoretinal Lymphoma: Vitreous and Serum MicroRNA Profiles Distinct from Uveitis. J Clin Med 2020;9:1844. [Crossref] [PubMed]
- de la Fuente MI, Alderuccio JP, Reis IM, et al. Bilateral Radiation Therapy Followed by Methotrexate-Based Chemotherapy for Primary Vitreoretinal Lymphoma Am J Hematol 2019;94:455-60. [PubMed]
- Marchese A, Agarwal A, Miserocchi E, et al. Features of Retinitis-like Lesions in Vitreoretinal Lymphoma. Ocul Immunol Inflamm 2019; Epub ahead of print. [Crossref] [PubMed]
- Fardeau C, Lee CP, Merle-Béral H, et al. Retinal fluorescein, indocyanine green angiography, and optic coherence tomography in non-Hodgkin’s primary intraocular lymphoma. Am J Ophthalmol 2009;147:886-894, 894.e881.
- Abusamra K, Oray M, Ebrahimiadib N, et al. Intraocular lymphoma: descriptive data of 26 patients including clinicopathologic features, vitreous findings, and treatment outcomes. Ocul Immunol Inflamm 2016;20:1-6.
- Hoffman PM, Mckelvie P, Hall AJ, et al. Intraocular lymphoma: a series of 14 patients with clinicopathological features and treatment outcomes. Eye 2003;17:513-21. [Crossref] [PubMed]
- Dalvin LA, Lim LAS, Ancona-Lezama D, et al. Tumor Control and Visual Acuity Outcomes in Vitreoretinal Lymphoma With and Without Sub-Retinal Pigment Epithelium Infiltration: Analysis of 125 Eyes of 70 Patients at a Single Ocular Oncology Center. Ophthalmol Retina 2019;3:998-1005. [Crossref] [PubMed]
- Dalvin LA, Lim LAS, Ancona-Lezama D, et al. Clinical Features Predictive of Survival in Patients With Vitreoretinal Lymphoma: Analysis of 70 Patients at a Single Ocular Oncology Center. Asia Pac J Ophthalmol (Phila) 2020;9:110-6. [Crossref] [PubMed]
- Barry RJ, Tasiopoulou A, Murray PI, et al. Characteristic optical coherence tomography findings in patients with primary vitreoretinal lymphoma: a novel aid to early diagnosis. Br J Ophthalmol 2018;102:1362-6. [Crossref] [PubMed]
- Marchese A, Miserocchi E, Giuffrè C, et al. Aurora borealis and string of pearls in vitreoretinal lymphoma: patterns of vitreous haze. Br J Ophthalmol 2019;103:1656-9. [Crossref] [PubMed]
- Herrlinger U. Primary CNS lymphoma: findings outside the brain. J Neurooncol 1999;43:227-30. [Crossref] [PubMed]
- Kawai N, Miyake K, Yamamoto Y, et al. 18F-FDG PET in the Diagnosis and Treatment of Primary Central Nervous System Lymphoma. Biomed Res Int 2013;2013:247152 [Crossref] [PubMed]
- Makino K, Hirai T, Nakamura H, et al. Does adding FDG- PET to MRI improve the differentiation between primary cerebral lymphoma and glioblastoma? Observer performance study. Ann Nucl Med 2011;25:432-8. [Crossref] [PubMed]
- Wilson DJ, Braziel R, Rosenbaum JT. Intraocular Lymphoma: Immunopathologic Analysis of Vitreous Biopsy Specimens. Arch Ophthalmol 1992;110:1455-8. [Crossref] [PubMed]
- Hwang CS, Yeh S, Bergstrom CS, et al. Diagnostic Vitrectomy for Primary Intraocular Lymphoma: When, Why, How? Int Ophthalmol Clin 2014;54:155-71. [Crossref] [PubMed]
- Tang PH, Karkhur S, Nguyen QD. Obtaining undiluted vitreous sample using small gauge pars plana vitrectomy and air infusion. Am J Ophthalmol Case Rep 2020;19:100768 [Crossref] [PubMed]
- Margolis R. Diagnostic vitrectomy for the diagnosis and management of posterior uveitis of unknown etiology. Curr Opin Ophthalmol 2008;19:218-24. [Crossref] [PubMed]
- Coupland SE. Analysis of Intraocular Biopsies. Dev Ophthalmol 2012;49:96-116. [Crossref] [PubMed]
- Cassoux N, Giron A, Bodaghi B, et al. IL-10 Measurement in Aqueous Humor for Screening Patients with Suspicion of Primary Intraocular Lymphoma. Invest Ophthalmol Vis Sci 2007;48:3253-9. [Crossref] [PubMed]
- Sugita S, Takase H, Sugamoto Y, et al. Diagnosis of intraocular lymphoma by polymerase chain reaction analysis and cytokine profiling of the vitreous fluid. Jpn J Ophthalmol 2009;53:209-14. [Crossref] [PubMed]
- Wolf LA, Reed GF, Buggage RR, et al. Vitreous cytokine levels. Ophthalmology 2003;110:1671-2. [Crossref] [PubMed]
- Costopoulos M, Touitou V, Golmard JL, et al. ISOLD: A New Highly Sensitive Interleukin Score for Intraocular Lymphoma Diagnosis. Ophthalmology 2016;123:1626-8. [Crossref] [PubMed]
- Kuo DE, Wei MM, Knickelbein JE, et al. Logistic Regression Classification of Primary Vitreoretinal Lymphoma Versus Uveitis by Interleukin 6 and Interleukin 10 Levels. Ophthalmology 2020;127:956-62. [Crossref] [PubMed]
- Takeda A, Hasegawa E, Nakao S, et al. Vitreous levels of interleukin-35 as a prognostic factor in B-cell vitreoretinal lymphoma. Sci Rep 2020;10:15715. [Crossref] [PubMed]
- Davis JL, Miller DM, Ruiz P. Diagnostic testing of vitrectomy specimens. Am J Ophthalmol 2005;140:822-9. [Crossref] [PubMed]
- Narasimhan S, Joshi M, Parameswaran S, et al. MYD88 L265P mutation in intraocular lymphoma: A potential diagnostic marker. Indian J Ophthalmol 2020;68:2160-5. [Crossref] [PubMed]
- Yonese I, Takase H, Yoshimori M, et al. CD79B mutations in primary vitreoretinal lymphoma: Diagnostic and prognostic potential Eur J Haematol 2019;102:191-6. [Crossref] [PubMed]
- Takhar JS, Doan TA, Gonzales JA. Primary vitreoretinal lymphoma: empowering our clinical suspicion Curr Opin Ophthalmol 2019;30:491-9. [Crossref] [PubMed]
- Choi S, Shin S, Lee ST, et al. Serial Detection of MYD88 L265P Mutation in the Aqueous Humor of a Patient with Vitreoretinal Lymphoma for Disease Monitoring. Ocul Immunol Inflamm 2020; Epub ahead of print. [Crossref] [PubMed]
- DeAngelis LM. Primary central nervous system lymphomas. Curr Treat Options Oncol 2001;2:309-18. [Crossref] [PubMed]
- Stefanovic A, Davis J, Murray T, et al. Treatment of isolated primary intraocular lymphoma with high-dose methotrexate-based chemotherapy and binocular radiation therapy: a single- institution experience Br J Haematol 2010;151:103-6. [Crossref] [PubMed]
- Baron M, Belin L, Cassoux N, et al. Temozolomide is effective and well tolerated in patients with primary vitreoretinal lymphoma. Blood 2020;135:1811-5. [Crossref] [PubMed]
- Hashida N, Ohguro N, Nishida K. Efficacy and complications of intravitreal rituximab injection for treating primary vitreoretinal lymphoma. Transl Vis Sci Technol 2012;1:1. [Crossref] [PubMed]
- Rosenfeld MR, Pruitt AA. Management of malignant gliomas and primary CNS lymphoma: standard of care and future directions. Continuum 2012;18:406-15. [PubMed]
- Kvopka M, Lake SR, Smith JR. Intraocular chemotherapy for vitreoretinal lymphoma: A review. Clin Exp Ophthalmol 2020;48:240-8. [Crossref] [PubMed]
- Zhou X, Zhou X, Shi H, et al. Reduced frequency of Intravitreal methotrexate injection lowers the risk of Keratopathy in Vitreoretinal lymphoma patients. BMC Ophthalmol 2020;20:189. [Crossref] [PubMed]
- Cicinelli MV, Marchese A, Miserocchi E, et al. Retinal and Choroidal Changes of Vitreoretinal Lymphoma from Active to Remission Phase after Intravitreal Rituximab. Ocul Immunol Inflamm 2020;28:637-46. [Crossref] [PubMed]
- Holdhoff M, Mrugala MM, Grommes C, et al. Challenges in the Treatment of Newly Diagnosed and Recurrent Primary Central Nervous System Lymphoma. J Natl Compr Canc Netw 2020;18:1571-8. [Crossref] [PubMed]
- Choi YS. Recent advances in the management of primary central nervous system lymphoma. Blood Res 2020;55:S58-S62. [Crossref] [PubMed]
- Zhang Y, Zhou DB. Primary central nervous system lymphoma: status and advances in diagnosis, molecular pathogenesis, and treatment. Chin Med J (Engl) 2020;133:1462-9. [PubMed]
- Ferreri AJ, Cwynarsky K, Pulczynski E, et al. International extranodal lymphoma study group (IELSG). Chemoimmunotherapy with methotrexate, cytarabine, thiotepa and rituximab (MATRix regimen) in patients with primary CNS lymphoma: results of the first randomization of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol 2016;3:e217-e227. [Crossref] [PubMed]
- Ferreri AJ, Holdhoff M, Nayak L, et al. Evolving Treatments for Primary Central Nervous System Lymphoma. Am Soc Clin Oncol Educ Book 2019;39:454-66. [Crossref] [PubMed]
- Yang H, Xun Y, Yang A, et al. Advances and challenges in the treatment of primary central nervous system lymphoma. J Cell Physiol 2020;235:9143-65. [Crossref] [PubMed]
- Ugonma N. Chukwueke, Lakshmi Nayak. Central Nervous System Lymphoma Hematol Oncol Clin North Am 2019;33:597-611. [Crossref]
- Soussain C, Choquet S, Blonski M, et al. Ibrutinib monotherapy for relapse or refractory primary CNS lymphoma and primary vitreoretinal lymphoma: Final analysis of the phase II ‘proof-of-concept’ iLOC study by the Lymphoma study association (LYSA) and the French oculo-cerebral lymphoma (LOC) network. Eur J Cancer 2019;117:121-30. [Crossref] [PubMed]
- Treon SP, Tripsas CK, Meid K, et al. Ibrutinib in previously treated Waldenström’s macroglobulinemia. N Engl J Med 2015;372:1430-40. [Crossref] [PubMed]
- Ferreri AJ, Reni M. Prognostic Factors in Primary Central Nervous System Lymphomas. Hematol Oncol Clin North Am 2005;19:629-49. [Crossref] [PubMed]
- Char DH, Ljung BM, Miller T, et al. Primary intraocular lymphoma (ocular reticulum cell sarcoma) diagnosis and management. Ophthalmology 1988;95:625-30. [Crossref] [PubMed]
Cite this article as: Giuffrè C, Menean M, Modorati GM, Marchese A, Cicinelli MV, Bandello F, Miserocchi E. Primary vitreoretinal lymphoma: recent advances and literature review. Ann Lymphoma 2020;4:17.


