
Epidemiology of marginal zone lymphoma
Introduction
Marginal zone lymphoma (MZL) is an indolent subtype of non-Hodgkin lymphoma (NHL) with an estimated 7,460 new cases diagnosed in the US in 2016, which represents 7% of all mature NHL (1). There are three recognized subtypes of MZL. Extranodal MZL (EMZL) of mucosa-associated lymphoid tissue (MALT) was first described in 1983 (2), and was included in the REAL Classification in 1994 (3), while the other two subtypes, nodal MZL (NMZL) and splenic MZL (SMZL), were added as entities in the World Health Organization (WHO) classification for hematologic and lymphoma tissues in 2001 (4). All three entities were included in the International Classification of Diseases for Oncology, Third Edition (ICD-O-3)(5) which was published in 2000 and has been used by the Surveillance, Epidemiology and End Results (SEER) Program to generate US statistics since 2001. Diagnosis and classification of MZL subtypes can be challenging in practice due to frequent mixed clinical scenarios. In a large French study, the concordance between referral and expert review was relatively high for MALT lymphoma (83.0%) and SMZL (79.6%), but lower for NMZL (59.2%), with the latter changing from a low-grade B-NHL to NMZL in 88% of patients (6). A nested classification of lymphoid neoplasms for epidemiologic research was developed in 2007 by the International Lymphoma Epidemiology (InterLymph) Consortium (7), and has been incorporated into the SEER statistics for descriptive epidemiology as well as used to facilitate pooling across epidemiology studies. The descriptive epidemiology data on MZL in the SEER database, which also underlies classification in many case-control and cohort studies, uses very specific coding rules to classify MZL and its subtypes (8), acknowledging challenges in coding from routine pathology and clinical records.
Given the relative recent identification of these entities, population-based data on incidence of MZL and epidemiologic studies to identify risk factors are in many ways still in their infancy. For example, epidemiologic studies prior to the late 1990s are unable to assess MZL, as they are distributed across a majority of the Working Formula categories (7) and would require re-review of all pathology. The current challenges in classification of MZL and its subtypes would also impact both the descriptive and analytic epidemiology findings. Further, until recently much of the literature focused on analyzing NHL as a single entity and NHL subtype associations were only a secondary objective. In addition, early case-control and cohort studies often did not have a large number of cases, and therefore had too few cases to assess associations with rarer subtypes like MZL. To overcome these issues, the InterLymph Consortium initiated the Subtypes Project in order to pool individual-level study data from 20 case-control studies (17,471 cases and 23,096 controls) in order to examine a range of risk factors for each of the major NHL subtypes, including 1,052 MZL cases (EMZL n=633, NMZL n=157, and SMZL n=140), and then to systemically evaluate risk factor patterns across subtypes (9).
As a comprehensive review of the epidemiology of NHL was recently published (10), this review first focuses on the descriptive epidemiology of MZL, including incidence by age, sex, race/ethnicity, and then on the analytic epidemiology that identifies genetic and environmental risk factors for MZL.
Descriptive epidemiology
Incidence
Using data from the SEER-18 program (11), the age-standardized incidence rate (using the US year 2000 as the standard population) for MZL from 2001–2017 was 19.6 per 1,000,000, and was slightly higher in males (20.5) compared to females (19.1), with an incidence rate ratio (IRR) of 1.07 (Table 1). EMZL represents 60.8% of MZLs, followed by NMZL (30.3%) and SMZL (8.9%) in the SEER data. Both SMZL (IRR: 1.17) and NMZL (IRR: 1.15) were slightly more common in males, while the incidence rates for males and females were nearly identical for NMZL (IRR: 1.02). For EMZL, the most common anatomic sites were the stomach (30%), followed by eye/adnexa (12%), skin (10%), lung (9%) and salivary gland (7%). The male to female ratio was most skewed to males for NMZL of the skin (IRR: 1.47), nasopharynx (IRR: 1.33), small intestine (IRR: 1.32) and colorectum (IRR: 1.30) and was most skewed to females for breast (IRR: 0.06), salivary gland (IRR: 0.48), and thyroid (IRR: 0.56).
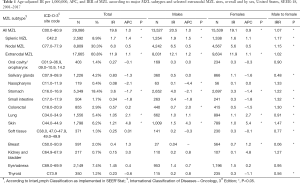
Full table
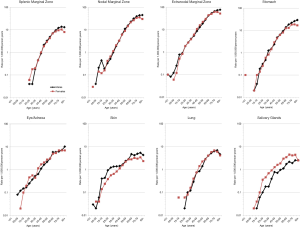
For each MZL subtype, the incidence increased sharply with age (Figure 1), similar to many other common cancers in adults, and suggests a role for cumulative lifetime exposures and increasing susceptibility with age. This pattern also holds for the 5 most common sites of EMZL, with the age-incidence curve steepest for EMZL of the stomach (Figure 1).
Overall, incidence rates (age standardized per 1,000,000) for MZL are highest in non-Hispanic whites (20.7), followed by Hispanics of all races (17.6), and then non-Hispanic blacks (15.4) and Asian/Pacific Islanders (15.0); this pattern held for SMZL, NMZL and EMZL (Table 2). Non-Hispanic whites had generally higher incidence rates for the various anatomic sites of EMZL, with the exception of higher incidence (IRR >1.2, P<0.05) of colorectal (IRR: 1.92), thyroid (IRR: 1.47), and eye/adnexa (IRR: 1.42) in Asian/Pacific Islanders; and salivary gland (IRR: 1.44) and colorectal (IRR: 1.32) in Hispanics of all races.
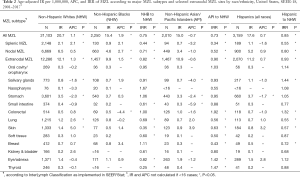
Full table
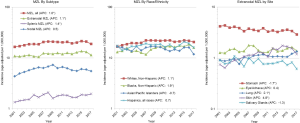
The US trends in incidence of MZL from 2001–2017 are shown in Figure 2, with a statistically significant increase in the age-adjusted incidence for MZL overall (+1.0% per year) and for SMZL (+1.4% per year) and EMZL (+1.1% per year), while the much smaller annual increase for NMZL (+0.5% per year) was not statistically significant. The time trends for MZL overall (Figure 2) were increasing for non-Hispanic whites (+1.1% per year) and blacks (+1.9% per year), while there was no statistically significant change for Asian/Pacific Islanders (−0.7% per year) or Hispanics (+0.7% per year). Of the most common EMZL sites, skin (+4.8% per year) and lung (+2.1% per year) were increasing, stomach (−1.7% per year) and salivary gland (−1.3% per year) were decreasing, and eye/adnexa was largely stable (Figure 2).
International comparisons of MZL rates are limited due to a general lack of data, and even when there are data, they need to be evaluated with caution due to differences in pathology review, cancer registration, case definition (including reporting of subtypes), timeframes, standard populations (used for incidence comparisons), and other factors; nevertheless, they can provide some insights. In a large international pathology study of the relative frequencies of NHL subtypes (excluding CLL) based on 4,539 consecutive cases from 26 centers in 24 countries (12), EMZL of MALT type accounted for a higher percentage in developed countries (8.8%) compared to developing countries (5.2%, P<0.05), while frequencies were similar for NMZL and SMZL combined in developed (3.0%) and developing (2.5%) countries. Compared to age-standardized MZL incidence rates (per 1,000,000) in the US for MZL for 2001–2009 (18.3) (13), MZL rates were lower in France (Cote d’Or) for 2000–2009 (15.0) (14), Australia for 1997–2006 (5.0) (15), Singapore for 2008–2012 (10.0) (16), and Japan for 2008 (5.0) (17), and higher in the UK for 2004–2012 (26.2) (18). Male-to-female IRRs for MZL from these studies ranged from 0.5 for Japan, 1.0 for Australia, 1.1 for the US, 1.3 for Singapore, 1.5 for France, and 1.6 for the UK, all of which are much smaller than that seen for most other NHL subtypes which tend to have a strong male predominance (10). MZL incidence rates were increasing in the US for 2001–2009 (13), France for 2000–2009 (14), Australia for 1997–2008 (15), Singapore for 1998–2012 (16) and Japan for 1993–2008 (17), with some but not all of the increase likely driven by better pathologic diagnosis of MZL over time.
Survival and mortality
Based on data from the SEER-18 Program for cases diagnosed 2000–2017 (11), the 5-year relative survival rate for MZL, which accounts for competing causes of death, was 89.8%, and was similar across racial/ethnic groups (Table 3). NMZL had the lowest survival (82.8%), with higher survival for SMZL (85.3%) and EMZL (93.8%), and survival rates were similar across race/ethnicity except for a lower survival for SMZL in Hispanics of all races (76.3%). Of EMZL sites, 5-year survival was highest for skin (100%) and lowest for small intestine (87.9%). While data for EMZL sites by race/ethnicity were limited, the most notable disparities were lower survival for colorectal and lung EMZL in non-Hispanic blacks and lung EMZL in Asian/Pacific Islanders. The 5-year relative survival rates by sex were very similar for most MZL subtypes and EMZL sites, with differences between sexes of more than 10% only observed for EMZL of the nasopharynx, breast, and kidney/bladder (Figure 3).
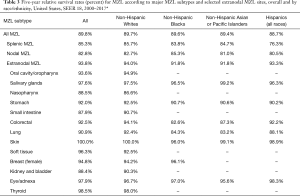
Full table
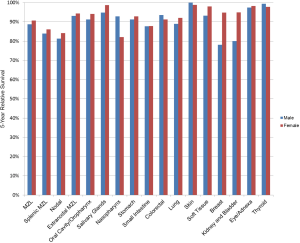
The 5-year relative survival rate for MZL in the UK was 77.2% for cases diagnosed 2004–2012, with little sex difference (18), and 80% in the Netherlands from 1989–2008, also similar by sex (19). Based on data from HAEMACARE (48 registries in 20 European countries), the estimated 5-year relative survival for MZL cases diagnosed 2000–2002 was 81.4%, with only minor differences by sex or region of Europe (20). In Singapore, 5-year age-standardized relative survival for MZL increased from 1998-2002 (76.4%) to 2003–2007 (80.8%) to 2008–2012 (86.7%), and while the male/female gap decreased, it was still higher in males (91.1%) compared to females (81.3%) for 2008–2012 (16). In the US from 1995–2009, 5-year relative survival for all MZL was 84.4% (21), and MZL overall survival improved slightly in the National Cancer Database (hospital-based registries that cover ~70% of US population) from 1998–2000 to 2004–2006 (22).
Family history and genetic susceptibility
In the InterLymph Subtypes Project, a family history of NHL in a first degree relative was associated with a 1.65-fold increased risk of MZL (95% CI: 1.10–2.46) and this association was not attenuated by adjustment for potential confounding factors (23). This risk estimate aligns with data from a pooled analysis of five Nordic registries from 1955–2010 (standardized incidence ratio (SIR) 1.6, 95% CI: 0.8–2.8) (24). The latter study also found elevated risk of MZL in persons with a first-degree relative with DLBCL (SIR 4.0, 95% CI: 1.5–8.7), while there were too few cases of other NHL subtypes to estimate risk of MZL. In the InterLymph Subtypes Project (23), risk was also elevated for a first-degree family history of leukemia (OR 1.66, 95% CI: 1.15–2.38) or Hodgkin lymphoma (HL, OR 2.73, 95% CI: 1.36–5.50), but not with multiple myeloma (OR 0.55, 95% CI: 0.13–2.37). For many common lymphoma subtypes, any family history of lymphoma is associated with an increased risk of that subtype, while a family history of a specific subtype tends to show the strongest risk association for that subtype, suggesting both a shared etiology across multiple subtypes as well as a role for subtype-specific genetic factors (25). For MZL, the former association is evident, while for the latter association there are no data for a family history of MZL as a risk factor for MZL per se, perhaps in part due to its fairly recent addition as a lymphoma subtype as well as its relative rarity. Finally, while familial aggregation represents both shared genetic and environmental factors, the findings to date suggest that the associations with family history, including risk of MZL, are not strongly confounded by non-genetic risk factors (26).
Genetic epidemiology studies are used to identify genetic loci. Linkage studies in families are most commonly used to identify genes with major susceptibility effects (e.g., Mendelian), but there are no linkage studies of MZL or most major NHL subtypes (25). The lack of major genes identified in MZL may be due to the relatively recent introduction of this subtype. But the lack of identification of major genes for MZL or any of the major lymphoma subtypes (including CLL, which has high hereditability) also raises the hypothesis that multiple, low to moderate risk variants that are common in the population (i.e., >5% minor allele frequency) may be more relevant in lymphoma etiology than single, highly penetrant variants that are very rare (26). To identify common genetic variants, the case-control study design is most commonly used, and either evaluates candidate genes (hypothesis driven) or the entire genome simultaneously via an agnostic (hypothesis-free) genome-wide association study (GWAS) approach (26). Prior to GWAS, there were no studies targeted specifically for MZL, and MZL was rarely reported as a subtype finding in studies of all NHL (25). In an InterLymph two-stage GWAS of 1,281 MZL cases and 7,127 controls of European ancestry (27), two independent loci in the HLA region were identified near HLA-B (rs2922994, P=2.43×10−9) and BTNL2 (rs9461741, P=3.95×10−15). Associations were slightly stronger for EMZL versus other MZL, but there was no evidence for heterogeneity (P>0.05). While no imputed HLA alleles reached genome-wide significance, HLA-B*08:01 and the HLA-B allele encoding an aspartic acid residue at position 9 (Asp9) in the HLA-B peptide binding grove were strongly associated with MZL and were in high linkage disequilibrium with the GWAS SNP rs2922994 near HLA-B (r2=0.97), and thus were not distinguishable from each other in conditional modelling. For HLA class II, there was a suggestive association with HLA-DRB1*01:02, which was correlated with the GWAS SNP rs9461741 near BTNL2 (r2=0.69), and these were also not distinguishable from each other in conditional modelling. However, HLA-B*08:01 and HLA-DRB1*01:02 were independent when included in the same model, which further supported independent loci for HLA class I and II loci with MZL risk.
The HLA region is one of the few genetic regions associated with multiple NHL subtypes, showing strong associations with DLBCL, follicular lymphoma (FL), CLL/SLL, HL, and natural killer/T-cell lymphoma (NKTCL), although specifics vary by subtype (25). Interestingly, GWAS-based analysis of MZL and three autoimmune diseases (rheumatoid arthritis, systemic lupus erythematosus, and multiple sclerosis) found only a few weakly-associated loci jointly shared and these loci explained little total risk, suggesting that common genetic variation may not be the primary explanation for the link between MZL and autoimmune disease.
In an analysis of HLA diversity and MZL risk using the InterLymph GWAS data, homozygosity at class I HLA-B (OR 1.34, 95% CI: 1.01–1.78) and HLA-C (OR 1.33, 95% CI: 1.04–1.70) and at class II HLA-DRB1 (OR 1.45, 95% CI: 1.12–1.89) were associated with increased risk of MZL (28). Similar associations were observed for DLBCL, while FL and CLL showed different patterns with HLA zygosity. These findings support the larger hypothesis that homozygosity at HLA loci reduces the diversity of peptides that can be presented, reflecting the ability to respond to infectious agents, autoimmune conditions, or cancerous cells, and is thus of relevance for MZL etiology.
Infectious agents
Helicobacter pylori
The International Agency for Research on Cancer (IARC) has classified H. pylori as carcinogenic to humans (Group 1) with sufficient evidence that they cause low-grade B-cell MALT gastric lymphomas in humans (29). H. pylori is a gram-negative bacterium, and infection can range from asymptomatic to the development of gastritis associated with mucosal atrophy, ulceration, and gastric cancer, which includes gastric adenocarcinoma as well as the less common (<5%) gastric MALT lymphoma. The evidence of a causal role of H. pylori in gastric MALT lymphoma includes isolation of H. pylori from a majority of MALT lymphomas (30); epidemiologic study showing a strong association of prior infection with risk (31); isolation of the B-cell lymphoma clone in the chronic gastritis that preceded the MALT lymphoma (32); and eradication of H. pylori with antibiotics and proton pump inhibitors leading to regression of gastric MALT lymphoma in over 75% of cases and subsequent long-term survival (33). In the InterLymph Subtypes Project, peptic ulcer was associated with MZL (OR 1.51, 95% CI: 1.21–2.03), and this was specific to EMZL (OR 1.83, 95% CI: 1.35–2.49) and was not associated with NMZL or SMZL (23). In the SEER-Medicare study of 4,289 MZL cases and 200,000 controls, gastric ulcer was associated with increased risk of MZL (OR 1.55, 95% CI: 1.28–1.88), but not risk of DLBCL, CLL/SLL, FL, or TCL (34). The pathogenic mechanism underling this association likely initially involves chronic inflammation, lymphocyte infiltration, antigenic stimulation, direct effects of B-cell stimulation, and perhaps oxidative stress; later events include accumulation of genetic abnormalities (decreasing the role of antigenic stimulation) and histologic transformation (33). There is evolving evidence of strain pathogenicity (e.g., stains expressing cytotoxin-associated gene A (CagA) protein are associated with MALT lymphoma) and interaction with host genetic susceptibility (35) .
Hepatitis viruses
Hepatitis C virus (HCV) is an RNA virus that is both hepatotropic and lymphotropic and is typically transmitted through blood. IARC has also classified HCV as a Group 1 carcinogen and causally associated with B-cell lymphomas (29). HCV infection was associated with MZL (OR 3.41; 95% CI: 2.39–4.87) in a meta-analysis of 220 MZL cases and 3,003 controls from 5 studies (36), and this association was also observed in a SEER-Medicare study of 1,908 MZL and over 120,000 controls (OR 2.20; 95% CI: 1.22–3.95) (37). In an InterLymph pooled analysis of 383 MZL cases and 6,269 controls, HCV seropositivity was associated with MZL (OR 2.47; 95% CI: 1.44–4.23) (38). In the InterLymph Subtypes Project, HCV seropositivity was most strongly associated with EMZL (OR 5.29, 95% CI: 2.48–11.28) and NMZL (OR 4.36, 95% CI: 0.54–35.38) but not SMZL (OR 0.84, 95% CI: 0.11–6.28) (23). In support of a potential causative association, all 9 patients with HCV+ SMZL had disease regression after treatment with INFα and ribavirin (39); this initial observation has been extended, with treatment leading to remission in ~75% of HCV+ MZL cases of various subtypes (33). While the underlying pathobiology remains to be fully determined, the leading hypothesis is thought to be chronic inflammation, chronic antigenic B-cell stimulation, and B-cell clonal expansion, while evidence for B-lymphocyte infection is mixed and a direct transforming role has not been shown (40).
Hepatitis B virus (HBV) is a small DNA virus that was judged in a 2009 evaluation by IARC to be a Group 1 carcinogen, but to have only limited evidence of a causal link with NHL (29). In a meta-analysis of 17 case-control and 5 cohort studies (over 40,000 NHL cases), HBV infection was associated with an increased risk of NHL overall (OR 2.24, 95% CI: 1.80–2.78), but MZL was not assessed (41). In the SEER-Medicare study, HBV did not show a statistically significant elevation in risk of MZL (OR 1.51, 95% CI: 0.71–3.22) (37).
Human immunodeficiency virus (HIV)
HIV is classified as a Group 1 carcinogen by IARC with sufficient evidence in humans that it causes NHL in humans (29). While MZL is not an AIDS-defining lymphoma subtype, risk of MZL (SIR 2.4, 95% CI: 1.6–3.4) was elevated in HIV/AIDS relative to the general population, with similar risks in people with HIV-only and with AIDS and in pre- and post-HAART eras (42), suggesting mechanisms other than immunodeficiency (driver of AIDS-defining lymphoma subtypes) and raising the more likely mechanism of chronic antigenic stimulation by HIV in MZL pathogenesis.
Chlamydia psittaci (C. psittaci)
C. psittaci is an obligate intracellular bacterium usually found in animals (e.g., birds, cats and other pets) and is spread to humans through inhalation of aerosolized bacteria or handling contaminated feathers or fecal matter with significant geographic variability (43). It has been detected in a variety of B-cell lymphomas including EMZL of the lungs, skin, thyroid and salivary glands (35), but it is most strongly linked to ocular adnexal MALT lymphomas, with an Italian series detecting C. psittaci DNA in 80% of cases compared to nonneoplastic orbital biopsies (0%) or reactive lymphadenopathy samples (12%) (44). Treatment of cases with doxycycline has an overall remission rate of 48% summarized across 7 reports, adding further evidence for a causal role (33). Mechanistically, C. psittaci is thought to create an ongoing inflammatory response, where auto-antigens could be exposed and presented, driving proliferation and expansion of self-reactive B cells (45).
Borrelia burgdorferi (B. burgdorferi)
B. burgdorferi, a bacterium that is the causative agent of Lyme disease, is transmitted to humans by tick bite. The correlation of a skin manifestation of Lyme disease (acrodermatitis chronica atrophicans) and cutaneous B-cell lymphomas led to the initial evaluation of B. burgdorferi as a potential causative factor (46). B. burgdorferi infection varies widely by geography, with higher rates in endemic areas (e.g., parts of North American and Central Europe), where it can be detected in 10% to 40% of patients with cutaneous MZL (35). Remission after antibiotic treatment has been documented in anecdotal case reports (33). A large case-control study did not observe an association of tick bite, or presence of anti-Borrelia antibodies with risk of MZL overall (results for cutaneous MZL not reported) (47). Mechanistic parallels with H. pylori have been noted, with the infection driving chronic antigenic stimulation resulting in infiltration of the dermis by lymphocytes and formation of germinal centers (33).
Campylobacter jejuni (C. jejuni)
Immunoproliferative small intestinal disease (IPSID) is a rare MALT lymphoma subtype endemic in the Mediterranean region and the Middle East (35). It can be managed with sustained antibiotics in a majority of patients, while untreated patients can undergo histologic transformation to DLBCL (33). The pathogenesis of IPSID is thought to be chronic antigenic stimulation of the intestinal immunoglobulin A secretory immune system, further suggesting an infectious etiology (48). An initial case series (49) and a confirmatory case report (50) identified C. jejuni, a bacterium usually carried by birds that causes food-borne infections in humans, in the biopsy samples of 5 of 8 patients with IPSID; two patients treated with antibiotics responded. Whether this is a causal association remains to be determined (35).
Other infectious agents
Achromobacter xylosoxidans (A. xylosoxidans), a gram-negative betaproteo-bacterium, has been suggested as an etiologic agent in pulmonary MALT lymphomas based on a study from six European countries that reported detection of A. xylosoxidans in 46% of 124 cases compared to 18% of 82 control tissues (51). Further studies are needed to establish a casual association.
Other biologic agents classified by IARC as Group 1 carcinogens and linked to lymphomas are Epstein-Barr virus (EBV), Kaposi sarcoma herpes virus (KSHV), and Human T-cell lymphotropic virus, type 1 (HTLV-1) (29). EBV is causally linked to Burkitt lymphoma (BL), extranodal NKTCL, nasal type and NHL in the context of immunosuppression, but not to MZL (10). Infectious mononucleosis was not associated with risk of MZL in an InterLymph pooling project (OR 0.97, 95% CI: 0.64–1.47) (52). HTLV-1 is linked to adult T-cell leukemia and lymphoma and not to other lymphoma subtypes (10). KSHV is a causal agent in primary effusion lymphoma (29), and in a case-control study was linked with lymphoplasmacytic and low-grade B-cell lymphoma NOS, but was not associated with MZL (53).
Human Pegivirus (HPgV) is a single-stranded RNA virus transmitted through parenteral, sexual, and perinatal routes. In a study of 184 MZL cases and 1,572 controls, HPgV viremia was associated with elevated risk of MZL after adjusting for multiple NHL risk factors (OR 1.93, 95% CI: 1.03–3.63) (54), while a smaller case-control study (75 MZL cases) found no association (OR 1.02, 95% CI: 0.05–6.03) (55).
Medical history risk factors
Autoimmune conditions
In an InterLymph pooling project (56), risk of MZL was strongly associated with Sjögren syndrome (OR 30.6, 95% CI: 12.3–76.1), particularly secondary SS (OR 44.6, 95% CI: 10.6–187). Of MZL of known anatomic sites, Sjögren syndrome was associated with a 1,000-fold risk of parotid gland MALT lymphoma (OR 996, 95% CI: 216–4,596). Systemic lupus erythematosus (SLE) was also associated with increased risk of MZL (OR 7.52, 95% CI: 3.39–16.7), while there were weaker elevated risks with pernicious anemia (OR 2.29, 95% CI: 0.58–8.94), Crohn disease (OR 2.41, 95% CI: 0.69–8.46), and hemolytic anemia (OR 2.23, 95% CI: 0.24–21.0), although none of the latter estimates were statistically significant. Finally, MZL was not associated with psoriasis, ulcerative colitis, type 1 diabetes, sarcoidosis, or multiple sclerosis. Chronic autoimmune (Hashimoto) thyroiditis has been associated with a 67-fold risk of primary thyroid lymphoma (95% CI: 40–110) (57), most commonly as a MALT lymphoma or high-grade lymphoma transformed from a MALT lymphoma (58). In the large SEER-Medicare study of 1,908 MZL cases and >120,000 controls (59), MZL was associated with Sjögren syndrome (OR 6.6, 95% CI: 4.6–9.5), SLE (OR 2.8, 95% CI: 1.7–4.7), hemolytic anemia (OR 7.4, 95% CI: 3.1–18), celiac disease (OR 3.5, 95% CI: 1.3–9.8), pernicious anemia (OR 1.4, 95% CI: 1.0–1.8), and rheumatoid arthritis (RA, OR 1.2, 95% CI: 1.0–1.6), while there was no association with 19 other autoimmune conditions including Crohn disease and Hashimoto thyroiditis (no data on thyroid EMZL per se). In the InterLymph Subtypes Project (23), MZL was associated with autoimmune conditions classified as B-cell (OR 5.75, 95% CI: 3.11–8.33) but not T-cell (OR 1.05, 95% CI: 0.72–1.52) activating, consistent with the strong associations with Sjögren syndrome and SLE, and these associations extended to each of the EMZL, NMZL, and SMZL subtypes. While it is challenging to disentangle the effects of disease, treatments (which are often immunosuppressive, and some are cytotoxic), and host factors (including shared genetics or environmental factors), current understanding is that level of inflammation and disease severity are the major drivers of lymphoma development via direct antigenic stimulation by microbial or auto-antigens, indirect immune stimulation by chronic inflammation, or molecular changes leading to constitutive B-/T-cell activation, which are specific to each autoimmune/inflammatory condition (60).
Solid organ transplantation
Solid organ transplantation includes an early, intense induction phase of immunosuppression followed by a later maintenance phase and long-term chronic immune dysfunction predisposing to the development of lymphoma (61). In a large cohort, solid organ transplant recipients had a 6-fold overall excess risk of lymphoma compared to the general population (SIR 6.2; 95% CI: 5.9–6.5) (62). The excess risk was most strongly associated with a distinct spectrum of aggressive NHL subtypes (e.g., DLBCL, BL, and several T-cell lymphomas; SIRs 10–100), but there was also a moderate elevation in risk for MZL (SIR 2.2, 95% CI: 1.6–3.1), which was restricted to EMZL (SIR 2.8, 95% CI: 1.9–4.0) and not elevated for SMZL/NMZL (although only based on 6 cases). Elevated risks for MZL were observed across the age spectrum, time since transplantation, and type of transplanted organ. In the SEER-Medicare study, solid organ transplantation was strongly associated with DLBCL (OR 4.27, 95% CI: 3.23–5.64) and TCL (OR 3.58, 95% CI: 1.82–7.03), and more modestly with MZL (OR 1.92, 95% CI: 0.97–3.79) (34). The WHO recognizes EBV-positive MALT, but EBV negative MALT is not recognized as PTLD (63). The magnitude and pattern of risk supports a role immunosuppression impacting microbial activity and leading to chronic antigenic stimulation at commonly affected EMZL sites.
Atopy
In the InterLymph Subtypes Project, a history of asthma without other atopy (i.e., allergy, hay fever, eczema) was associated with an increased risk of MZL (OR 1.42, 95% CI: 1.03–1.97) compared to no history of asthma or atopic conditions; people with asthma and an atopic condition were not at elevated risk (OR 0.93, 95% CI: 0.70–1.25) (23). In an exploratory analysis by MZL subtype, eczema without other atopy was associated with SMZL (OR 2.28, 95% CI: 1.23–4.23) and EMZL (OR 1.50, 95% CI: 1.00–2.24) but not NMZL (23). More data will be required to confirm these associations, particularly study designs that can minimize recall bias and reverse causality (10).
Environmental risk factors
While there is an extensive literature on the role of occupational exposures, pesticides, industrial solvents and other environmental contaminants in NHL etiology (10), there are relatively few data addressing MZL. A German study found that total chlorinated hydrocarbons were associated with MZL (OR 7.0 for >47.3 vs. 0 ppm*years, 95% CI: 1.8–26.3), but that aromatic hydrocarbons (benzene, toluene, xylene, or styrene) were not (64). In 2012, IARC classified trichloroethylene (most common chlorinated hydrocarbon) as a Group 1 carcinogen, mainly based on findings related to kidney cancer, as evidence for it causing NHL was considered limited (65). Occupational exposure to breeding and/or slaughtering animals was associated with MZL compared to nodal NHL (OR 7.69, 95% CI: 2.65–22.34) (66). In the InterLymph Subtypes Project (23), of 32 occupations evaluated, only work as a general carpenter (OR 2.34, 95% CI: 1.23–4.45) or a teacher (OR 0.50, 95% CI: 0.35–0.70) were significantly associated with MZL, and these associations did not vary by MZL subtype. Subtype-specific associations were noted for occupation as a metal worker and NMZL (OR 3.56, 95% CI: 1.67–7.58) and as a general laborer and SMZL (OR 2.10; 95% CI: 1.15–3.84).
Also, from the InterLymph Subtypes Project (23), ever use of hair dye was not associated with risk of MZL among women (OR 1.17, 95% CI: 0.85–1.61), while in the subtype analysis it was associated with SMZL (OR 6.54, 95% CI: 1.53–27.9) but not EMZL or NMZL. Use of permanent hair dyes and dyes before 1980 (more carcinogenic formulations) were associated with the greatest risk of SMZL. This finding, based on a relatively small number of cases, requires further evaluation.
MZL risk was inversely associated with recreational sun exposure (OR 0.68 for highest vs. lowest quartile, 95% CI: 0.54–0.85) in the InterLymph Subtypes Project, which was not confounded by other risk factors (23); trends were apparent for EMZL and SMZL but not NMZL. In a meta-analysis (67), dietary vitamin D intake was not associated with MZL risk, while there were no published studies evaluating serum/plasma 25-hydroxyvitamin D with MZL risk, leaving unclear whether the suggested protective effect of sun exposure is mechanistically related to vitamin D or immunomodulation (10).
Lifestyle and behavioral risk factors
The InterLymph Subtypes Project evaluated smoking, alcohol use, anthropometrics, and physical activity with risk of MZL and MZL subtypes (23). While ever smoking cigarettes was not associated with risk of MZL or any MZL subtypes, duration of smoking was associated with MZL (OR 1.27 per category increase across categories of 0, 1–19, 20–29, 30–39, 40+ years, 95% CI: 1.03–1.57) (68). Use of any type of alcohol was inversely associated with MZL, and in particular use of wine, which showed a dose-response with g/week consumed (P-trend=0.002) and a 41% lower risk for the highest quartile of intake (OR 0.59 for >168 g/week vs. nondrinker, 95% CI: 0.37–0.95); these trends were apparent in the MZL subtypes. No associations were observed with height, usual adult body mass index, young adult body mass index, or physical activity.
Insights from NHL subtype risk factor patterns
InterLymph subtypes project
The InterLymph Subtypes Project, which included 11 NHL subtypes (abbreviations of MZL, BL, LPL/WM, DLBCL, CLL/SLL, FL, MCL, HCL, ALL, MF/SS, and PTCL defined in Table 4), formally evaluated whether a variety of medical, lifestyle, family history and occupational risk factors varied by NHL subtype as well as whether subtypes clustered based on risk factor profiles (68). With respect to etiologic heterogeneity (i.e., do risk factors vary by NHL subtype), Table 4 summarizes risk factors that were associated with 1 or more NHL subtypes (P<0.01 based on the ASSET test (69)), as well as the effect estimates (ORs) of each risk factor for NHL overall and for MZL, adjusted for age, sex, race/ethnicity, and study. With the exception of allergy, blood transfusion, height and smoking duration, MZL largely had a similar risk factor profile to NHL overall.
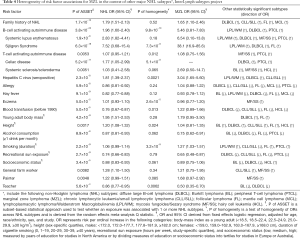
Full table
Next, a formal test was conducted to assess the heterogeneity of ORs for each significant exposure across NHL subtypes (Table 4), which was derived from the random effects meta-analysis Q statistic. For example, family history of NHL was strongly associated with 1 or more NHL subtypes (P=1.7×10−13) but there was no evidence for heterogeneity in the ORs (P=0.52) across subtypes: 8 of 11 subtypes showed ORs >1.5 (5 at P<0.05), including MZL. Homogenous risks across most subtypes were observed for family history of NHL, SLE, systemic sclerosis, allergy, hay fever, young adult BMI, alcohol use, recreational sun exposure, socioeconomic status, and occupations as a general farm worker or painter. In contrast, there was evidence for subtype heterogeneity of associations (P<0.05) for Sjögren syndrome, celiac disease, HCV seropositivity, eczema, blood transfusion before 1990, height, smoking duration, and occupation as a teacher.
A cluster analysis for subtypes was then conducted for each exposure showing significant heterogeneity across subtypes. MZL formed its own cluster for B-cell activating autoimmune disease (OR 5.46, 95% CI: 3.81–7.83), with a second cluster with weaker ORs formed by LPL/WM and DLBCL and a third cluster for all other subtypes. Similarly, MZL formed its own cluster for Sjögren syndrome (OR 38.1, 95% CI: 16.9–85.6), separating it from all other subtypes. For eczema, MZL clustered with all the other subtypes except MF/SS, which formed its own cluster; for HCV, MZL clustered with BL, LPL/WM and DLBCL; for blood transfusion prior to 1990, MZL clustered with LPL/WM and MF/SS; for height, MZL clustered with all the other subtypes except BL, which formed its own cluster; for smoking duration, MZL clustered with LPL/WM, FL, MCL, MF/SS and PTCL; and for occupation as a teacher, MZL clustered with LPL/WM and BL. Finally, while peptic ulcer disease did not achieve statistical significance by the ASSET test, it did show heterogeneity such that it was only associated with MZL (OR 1.5) and not any other NHL subtype. As observed in the genetics setting, there appear to be some risk factors that are more general across most subtypes and others that are only associated with MZL alone or MZL and a few other subtypes, although the latter associations varied by exposure.
Finally, to identify whether certain subtypes shared global risk factor profiles, hierarchical clustering was used based on an analysis of all risk factor data modeled simultaneously. The largest difference in risk factors profiles was between B- versus T-cell lymphomas, driven by eczema, occupation as a painter, T-cell activating autoimmune diseases, family history of multiple myeloma and cigarette smoking being more strongly associated with MF/SS and PTCL vs. all other B-cell subtypes. Within the B-cell node, the next split was for MZL and BL vs. all other B-cell subtypes, which was driven by Sjögren syndrome, occupation as a teacher, allergy, hay fever, alcohol use, systemic sclerosis, and HCV seropositivity. The remaining B-cell subtypes ultimately fell into four clusters: FL, MCL, LPL/WM and DLBCL, or CLL/SLL. Beyond the B vs. T cell split, this analysis also supports some shared risk factors across B-cell subtypes as well as subtype-specific risk factors.
Medical Condition-Wide Association Study (“MedWAS”)
In a MedWAS study utilizing SEER-Medicare data on 52,691 NHL cases and 200,000 controls, 5,296 medical conditions from claims data were comprehensively and agnostically evaluated for their association with each of five lymphoma subtypes (DLBCL, FL, CLL/SLL, MZL and TCL) (34). After accounting for multiple testing, 55 unique conditions were associated with 1 or more of the subtypes, but only 3 conditions—history of nonmelanoma skin cancer (ORs 1.19–1.55), actinic keratosis (ORs 1.12–1.25) and hemolytic anemia (ORs 1.64–4.07)—were associated with all five subtypes. Beyond those conditions, MZL was associated with 15 additional conditions, including previously reported associations with SLE and Sjögren syndrome (also associated with DLBCL); elevated sedimentation rate; and gastric ulcer. More novel findings needing replication included associations with thrombocytopenia (also with CLL/SLL, DLBCL, TCL); monoclonal paraproteinemia (also with CLL/SLL and DLBCL); aplastic anemia and anemia NOS; neutropenia; testicular hypofunction; benign prostatic hypertrophy; seborrheic keratosis (also with FL); and senile dementia, stroke (including TIA), and psychosis NOS (all 3 also associated with CLL/SLL, DLBCL, and FL).
Opportunities for prevention
The strongest opportunities for prevention of MZL currently lie in either preventing infections or treating them early. A striking example is the decline in northern Italy of H. pylori-associated gastric MALT lymphoma from 1997–2007, which the authors hypothesized was attributable to declining infection rates and earlier treatment of patients with peptic disease symptoms (70). While there is a strong link of autoimmune conditions with MZL, there is no clear way to prevent these conditions, and it is not clear that drugs used to reduce inflammatory activity lower lymphoma risk. For example, in RA the risk of lymphoma has not changed over time even in the setting of more intense treatments (71). In contrast, use of oral or intra-articular steroids in RA were associated with reduced risk of lymphoma that was most pronounced for DLBCL (72). Whether newer biologic agents used to treat both autoimmune conditions and lymphoma, such as anti-CD20 antibodies or newer drugs that target B-cell receptor pathways (e.g., tyrosine kinase inhibitors), ultimately decrease lymphoma risk among autoimmune patients is an important question yet to be evaluated (73). While definitive genetic loci have been identified for MZL, they are too limited and not yet sufficiently robust for screening in families or the population. While there are suggestive leads for modifiable environmental, lifestyle and behavioral risk factors that lend themselves to public health interventions (e.g., reducing exposure to trichloroethylene, reducing hair dye use, not smoking), other MZL risk factors present challenges to primary prevention approaches (e.g., protective effects of recreational sun exposure and moderate alcohol use), and none of these risk factors are established. New leads related to infections, environmental exposures, precursor conditions and immune biomarkers with MZL risk may provide future routes to prevention.
Conclusions and future directions
Progress on identifying MZL-specific risk factors has accelerated with the implementation of the InterLymph nested classification as well as with the availability of large pooled analyses. Established risk factors for MZL (or MZL subtypes) include family history of NHL, genetic loci in the HLA region, Helicobacter pylori infection (gastric MALT lymphoma), and several autoimmune diseases (Sjögren syndrome, systemic lupus erythematosus and Hashimoto thyroiditis), with strong evidence (but not definitive) for C. psittaci (ocular adnexal MALT lymphoma), B. burgdorferi (cutaneous MZL), hepatitis C virus, human immunodeficiency virus, and solid organ transplantation. There are also promising leads related to other infections, other B-cell activating autoimmune conditions, trichloroethylene exposure, certain occupations, hair dye, recreational sun exposure, smoking, and alcohol use, but these require further research including use of better exposure assessment, larger studies and stronger study designs.
MZL is a model of an antigen-driven malignancy, where environmental factors, tissue-specific factors, and host immune response (including impact of chronic inflammation and immunosuppression) drive lymphomagenesis. It is plausible that there may be additional pathogens relevant to MZL etiology, as well as additional environmental and lifestyle factors. We also remain early in our understanding of genetic susceptibility to MZL, and there are likely to be additional loci identified by GWAS and next generation sequencing. Deeper understanding of the striking role that HLA plays in MZL risk through the antigen processing and presentation is needed. Progress on all these fronts will also allow more robust evaluation of gene-environment interactions. Advances in the understanding of MZL biology and pathology, perhaps through better understanding of precursors and the genomic and epigenomic architecture of the MZL tumor genome, should also help identify novel links to specific risk factors. Finally, most studies have been conducted in Western populations of European ancestry, and future studies need to address racial/ethnic differences and other populations with contrasting environmental and infectious exposures.
Acknowledgments
The authors thank Ms. Sondra Buehler for editorial assistance.
Funding: This work was supported by the National Institutes of Health (P50 CA97274, U01 CA195568, R01 CA200703).
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Lymphoma for the series “Marginal Zone Lymphomas”. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aol-20-28). The series “Marginal Zone Lymphomas” was commissioned by the editorial office without any funding or sponsorship. TMH served as the unpaid Guest Editor of the series. JRC reports personal fees from Janssen, grants from NanoString, grants from BMS, non-financial support from Regeneron Genetics Center, grants from Genentech, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: Both authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Teras LR, DeSantis CE, Cerhan JR, et al. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin 2016;66:443-59. [Crossref] [PubMed]
- Isaacson P, Wright DH. Malignant lymphoma of mucosa-associated lymphoid tissue. A distinctive type of B-cell lymphoma. Cancer 1983;52:1410-6. [Crossref] [PubMed]
- Harris NL, Jaffe ES, Stein H, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood 1994;84:1361-92. [Crossref] [PubMed]
- Jaffe ES, Harris NL, Stein H, et al. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press, 2001.
- Fritz A, Percy C, Jack A, et al. International Classification of Diseases for Oncology: Third Edition. Geneva: World Health Organization, 2000.
- Laurent C, Baron M, Amara N, et al. Impact of Expert Pathologic Review of Lymphoma Diagnosis: Study of Patients From the French Lymphopath Network. J Clin Oncol 2017;35:2008-17. [Crossref] [PubMed]
- Morton LM, Turner JJ, Cerhan JR, et al. Proposed classification of lymphoid neoplasms for epidemiologic research from the Pathology Working Group of the International Lymphoma Epidemiology Consortium (InterLymph). Blood 2007;110:695-708. [Crossref] [PubMed]
- Ruhl J, Adamo M, Dickie L, et al. Hematopoietic and Lymphoid Neoplasm Coding Manual. Bethesda, MD: National Cancer Institute, 2020.
- Morton LM, Sampson JN, Cerhan JR, et al. Rationale and design of the International Lymphoma Epidemiology Consortium (InterLymph) non-Hodgkin Lymphoma Subtypes Project. J Natl Cancer Inst Monogr 2014;2014:1-14. [Crossref] [PubMed]
- Cerhan JR, Vajdic CM, Spinelli JJ. The non-Hodgkin Lymphomas. In: Thun MJ, Linet MS, Cerhan JR, et al., (editors). Schottenfeld and Fraumeni Cancer Epidemiology and Prevention. 4th ed. New York: Oxford University Press, 2018:767-96.
- Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence - SEER Research Data, 18 Registries, Nov 2019 Sub (2000-2017) - Linked To County Attributes – Time Dependent (1990-2017) Income/Rurality, 1969-2017 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2020, based on the November 2019 submission. Available online: www.seer.cancer.gov
- Perry AM, Diebold J, Nathwani BN, et al. Non-Hodgkin lymphoma in the developing world: review of 4539 cases from the International Non-Hodgkin Lymphoma Classification Project. Haematologica 2016;101:1244-50. [Crossref] [PubMed]
- Khalil MO, Morton LM, Devesa SS, et al. Incidence of marginal zone lymphoma in the United States, 2001-2009 with a focus on primary anatomic site. Br J Haematol 2014;165:67-77. [Crossref] [PubMed]
- Dandoit M, Mounier M, Guy J, et al. The heterogeneity of changes in incidence and survival among lymphoid malignancies in a 30-year French population-based registry. Leuk Lymphoma 2015;56:1050-7. [Crossref] [PubMed]
- van Leeuwen MT, Turner JJ, Joske DJ, et al. Lymphoid neoplasm incidence by WHO subtype in Australia 1982-2006. Int J Cancer 2014;135:2146-56. [Crossref] [PubMed]
- Lim RB, Loy EY, Lim GH, et al. Gender and ethnic differences in incidence and survival of lymphoid neoplasm subtypes in an Asian population: Secular trends of a population-based cancer registry from 1998 to 2012. Int J Cancer 2015;137:2674-87. [Crossref] [PubMed]
- Chihara D, Ito H, Matsuda T, et al. Differences in incidence and trends of haematological malignancies in Japan and the United States. Br J Haematol 2014;164:536-45. [Crossref] [PubMed]
- Smith A, Crouch S, Lax S, et al. Lymphoma incidence, survival and prevalence 2004-2014: sub-type analyses from the UK's Haematological Malignancy Research Network. Br J Cancer 2015;112:1575-84. [Crossref] [PubMed]
- van de Schans SA, van Steenbergen LN, Coebergh JW, et al. Actual prognosis during follow-up of survivors of B-cell non-Hodgkin lymphoma in the Netherlands. Haematologica 2014;99:339-45. [Crossref] [PubMed]
- Marcos-Gragera R, Allemani C, Tereanu C, et al. Survival of European patients diagnosed with lymphoid neoplasms in 2000-2002: results of the HAEMACARE project. Haematologica 2011;96:720-8. [Crossref] [PubMed]
- Olszewski AJ, Castillo JJ. Survival of patients with marginal zone lymphoma: analysis of the Surveillance, Epidemiology, and End Results database. Cancer 2013;119:629-38. [Crossref] [PubMed]
- Al-Hamadani M, Habermann TM, Cerhan JR, et al. Non-Hodgkin lymphoma subtype distribution, geodemographic patterns, and survival in the US: A longitudinal analysis of the National Cancer Data Base from 1998 to 2011. Am J Hematol 2015;90:790-5. [Crossref] [PubMed]
- Bracci PM, Benavente Y, Turner JJ, et al. Medical history, lifestyle, family history, and occupational risk factors for marginal zone lymphoma: the InterLymph Non-Hodgkin Lymphoma Subtypes Project. J Natl Cancer Inst Monogr 2014;2014:52-65. [Crossref] [PubMed]
- Fallah M, Kharazmi E, Pukkala E, et al. Familial risk of non-Hodgkin lymphoma by sex, relationship, age at diagnosis and histology: a joint study from five Nordic countries. Leukemia 2016;30:373-8. [Crossref] [PubMed]
- Cerhan JR, Braggio E, Slager SL, et al. Genetics in Lymphomagenesis. In: Wiernik PH, Dutcher JP, Gertz MA, editors. Neoplastic Diseases of the Blood. Sixth ed. New York: Springer, 2018:723-53.
- Cerhan JR, Slager SL. Familial predisposition and genetic risk factors for lymphoma. Blood 2015;126:2265-73. [Crossref] [PubMed]
- Vijai J, Wang Z, Berndt SI, et al. A genome-wide association study of marginal zone lymphoma shows association to the HLA region. Nat Commun 2015;6:5751. [Crossref] [PubMed]
- Wang SS, Carrington M, Berndt SI, et al. HLA Class I and II Diversity Contributes to the Etiologic Heterogeneity of Non-Hodgkin Lymphoma Subtypes. Cancer Res 2018;78:4086-96. [Crossref] [PubMed]
- Bouvard V, Baan R, Straif K, et al. A review of human carcinogens--Part B: biological agents. Lancet Oncol 2009;10:321-2. [Crossref] [PubMed]
- Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, et al. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet 1991;338:1175-6. [Crossref] [PubMed]
- Parsonnet J, Hansen S, Rodriguez L, et al. Helicobacter pylori infection and gastric lymphoma. N Engl J Med 1994;330:1267-71. [Crossref] [PubMed]
- Zucca E, Bertoni F, Roggero E, et al. Molecular analysis of the progression from Helicobacter pylori-associated chronic gastritis to mucosa-associated lymphoid-tissue lymphoma of the stomach. N Engl J Med 1998;338:804-10. [Crossref] [PubMed]
- Zucca E, Bertoni F, Vannata B, et al. Emerging role of infectious etiologies in the pathogenesis of marginal zone B-cell lymphomas. Clin Cancer Res 2014;20:5207-16. [Crossref] [PubMed]
- Engels EA, Parsons R, Besson C, et al. Comprehensive evaluation of medical conditions associated with risk of non-Hodgkin lymphoma using Medicare claims ("MedWAS"). Cancer Epidemiol Biomarkers Prev 2016;25:1105-13. [Crossref] [PubMed]
- Ferreri AJ, Govi S, Ponzoni M. Marginal zone lymphomas and infectious agents. Semin Cancer Biol 2013;23:431-40. [Crossref] [PubMed]
- Dal Maso L, Franceschi S. Hepatitis C virus and risk of lymphoma and other lymphoid neoplasms: a meta-analysis of epidemiologic studies. Cancer Epidemiol Biomarkers Prev 2006;15:2078-85. [Crossref] [PubMed]
- Anderson LA, Pfeiffer R, Warren JL, et al. Hematopoietic malignancies associated with viral and alcoholic hepatitis. Cancer Epidemiol Biomarkers Prev 2008;17:3069-75. [Crossref] [PubMed]
- de Sanjose S, Benavente Y, Vajdic CM, et al. Hepatitis C and non-Hodgkin lymphoma among 4784 cases and 6269 controls from the International Lymphoma Epidemiology Consortium. Clin Gastroenterol Hepatol 2008;6:451-8. [Crossref] [PubMed]
- Hermine O, Lefrere F, Bronowicki JP, et al. Regression of splenic lymphoma with villous lymphocytes after treatment of hepatitis C virus infection. N Engl J Med 2002;347:89-94. [Crossref] [PubMed]
- Marcucci F, Mele A. Hepatitis viruses and non-Hodgkin lymphoma: epidemiology, mechanisms of tumorigenesis, and therapeutic opportunities. Blood 2011;117:1792-8. [Crossref] [PubMed]
- Dalia S, Chavez J, Castillo JJ, et al. Hepatitis B infection increases the risk of non-Hodgkin lymphoma: a meta-analysis of observational studies. Leuk Res 2013;37:1107-15. [Crossref] [PubMed]
- Gibson TM, Morton LM, Shiels MS, et al. Risk of non-Hodgkin lymphoma subtypes in HIV-infected people during the HAART era: a population-based study. AIDS 2014;28:2313-8. [Crossref] [PubMed]
- Knittler MR, Sachse K. Chlamydia psittaci: update on an underestimated zoonotic agent. Pathog Dis 2015;73:1-15. [Crossref] [PubMed]
- Ferreri AJ, Guidoboni M, Ponzoni M, et al. Evidence for an association between Chlamydia psittaci and ocular adnexal lymphomas. J Natl Cancer Inst 2004;96:586-94. [Crossref] [PubMed]
- Dagklis A, Ponzoni M, Govi S, et al. Immunoglobulin gene repertoire in ocular adnexal lymphomas: hints on the nature of the antigenic stimulation. Leukemia 2012;26:814-21. [Crossref] [PubMed]
- Garbe C, Stein H, Dienemann D, et al. Borrelia burgdorferi-associated cutaneous B cell lymphoma: clinical and immunohistologic characterization of four cases. J Am Acad Dermatol 1991;24:584-90. [Crossref] [PubMed]
- Schöllkopf C, Melbye M, Munksgaard L, et al. Borrelia infection and risk of non-Hodgkin lymphoma. Blood 2008;111:5524-9. [Crossref] [PubMed]
- Brouet JC, Seligmann M. Letter: Selective IgA deficiency and idiopathic thrombocytopenic purpura. Lancet 1976;1:861-2. [Crossref] [PubMed]
- Lecuit M, Abachin E, Martin A, et al. Immunoproliferative small intestinal disease associated with Campylobacter jejuni. N Engl J Med 2004;350:239-48. [Crossref] [PubMed]
- Mesnard B, De Vroey B, Maunoury V, et al. Immunoproliferative small intestinal disease associated with Campylobacter jejuni. Dig Liver Dis 2012;44:799-800. [Crossref] [PubMed]
- Adam P, Czapiewski P, Colak S, et al. Prevalence of Achromobacter xylosoxidans in pulmonary mucosa-associated lymphoid tissue lymphoma in different regions of Europe. Br J Haematol 2014;164:804-10. [Crossref] [PubMed]
- Becker N, Falster MO, Vajdic CM, et al. Self-reported history of infections and the risk of non-Hodgkin lymphoma: an InterLymph pooled analysis. Int J Cancer 2012;131:2342-8. [Crossref] [PubMed]
- de Sanjosé S, Goedert JJ, Marshall V, et al. Risk of malignant lymphoma associated with human herpesvirus-8: a case-control study in Spain. Br J Cancer 2004;90:2145-8. [Crossref] [PubMed]
- Fama A, Xiang J, Link BK, et al. Human Pegivirus infection and lymphoma risk and prognosis: a North American study. Br J Haematol 2018;182:644-53. [Crossref] [PubMed]
- Krajden M, Yu A, Braybrook H, et al. GBV-C/hepatitis G virus infection and non-Hodgkin lymphoma: a case control study. Int J Cancer 2010;126:2885-92. [Crossref] [PubMed]
- Ekström Smedby K, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood 2008;111:4029-38. [Crossref] [PubMed]
- Holm LE, Blomgren H, Lowhagen T. Cancer risks in patients with chronic lymphocytic thyroiditis. N Engl J Med 1985;312:601-4. [Crossref] [PubMed]
- Pedersen RK, Pedersen NT. Primary non-Hodgkin's lymphoma of the thyroid gland: a population based study. Histopathology 1996;28:25-32. [Crossref] [PubMed]
- Anderson LA, Gadalla S, Morton LM, et al. Population-based study of autoimmune conditions and the risk of specific lymphoid malignancies. Int J Cancer 2009;125:398-405. [Crossref] [PubMed]
- Baecklund E, Smedby KE, Sutton LA, et al. Lymphoma development in patients with autoimmune and inflammatory disorders--what are the driving forces? Semin Cancer Biol 2014;24:61-70. [Crossref] [PubMed]
- Dierickx D, Habermann TM. Post-transplantation lymphoproliferative disorders in adults. N Engl J Med 2018;378:549-62. [Crossref] [PubMed]
- Clarke CA, Morton LM, Lynch C, et al. Risk of lymphoma subtypes after solid organ transplantation in the United States. Br J Cancer 2013;109:280-8. [Crossref] [PubMed]
- Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues (Revised 4th Edition). Lyon, France: IARC, 2017.
- Seidler A, Mohner M, Berger J, et al. Solvent exposure and malignant lymphoma: a population-based case-control study in Germany. J Occup Med Toxicol 2007;2:2. [Crossref] [PubMed]
- Guha N, Loomis D, Grosse Y, et al. Carcinogenicity of trichloroethylene, tetrachloroethylene, some other chlorinated solvents, and their metabolites. Lancet Oncol 2012;13:1192-3. [Crossref] [PubMed]
- Dolcetti R, Serraino D, Dognini G, et al. Exposure to animals and increased risk of marginal zone B-cell lymphomas of the ocular adnexae. Br J Cancer 2012;106:966-9. [Crossref] [PubMed]
- Park HY, Hong YC, Lee K, et al. Vitamin D status and risk of non-Hodgkin lymphoma: An updated meta-analysis. PLoS One 2019;14:e0216284. [Crossref] [PubMed]
- Morton LM, Slager SL, Cerhan JR, et al. Etiologic heterogeneity among non-Hodgkin lymphoma subtypes: the InterLymph Non-Hodgkin Lymphoma Subtypes Project. J Natl Cancer Inst Monogr 2014;2014:130-44. [Crossref] [PubMed]
- Bhattacharjee S, Rajaraman P, Jacobs KB, et al. A subset-based approach improves power and interpretation for the combined analysis of genetic association studies of heterogeneous traits. Am J Hum Genet 2012;90:821-35. [Crossref] [PubMed]
- Luminari S, Cesaretti M, Marcheselli L, et al. Decreasing incidence of gastric MALT lymphomas in the era of anti-Helicobacter pylori interventions: results from a population-based study on extranodal marginal zone lymphomas. Ann Oncol 2010;21:855-9. [Crossref] [PubMed]
- Hellgren K, Baecklund E, Backlin C, et al. Rheumatoid arthritis and risk of malignant lymphoma: is the risk still increased? Arthritis Rheumatol 2017;69:700-8. [Crossref] [PubMed]
- Hellgren K, Iliadou A, Rosenquist R, et al. Rheumatoid arthritis, treatment with corticosteroids and risk of malignant lymphomas: results from a case-control study. Ann Rheum Dis 2010;69:654-9. [Crossref] [PubMed]
- Smedby KE, Ponzoni M. The aetiology of B-cell lymphoid malignancies with a focus on chronic inflammation and infections. J Intern Med 2017;282:360-70. [Crossref] [PubMed]
Cite this article as: Cerhan JR, Habermann TM. Epidemiology of marginal zone lymphoma. Ann Lymphoma 2021;5:1.


