
Treatment of elderly patients with primary CNS lymphoma
Introduction
Primary central nervous system lymphoma (PCNSL) is a diffuse large B-cell lymphoma (DLBCL) confined to the brain. PCNSL shares with DLBCL one of the biggest issues in modern hematology: identifying the optimal care and treatment of older individuals (1,2). Akin to DLBCL, PCNSL has rising incidence with age and more importantly, lengthening of life expectancy has resulted in increasing prevalence of PCNSL in an older population (1,3).
The median age of presentation of PCNSL has generally been reported at around 65 years although real world data suggests this may be higher (2,4). The recently published French oculo-cerebral lymphoma network (LOC), a nationwide population-based study of 1,002 immunocompetent patients diagnosed between 2011 and 2016, reports 43% of patients aged >70 years (5). A combination of comorbidities, poor baseline performance status (PS) and potential drug toxicity are widely accepted as the main challenges when defining optimal therapeutic strategies for this age group (1,6,7).
The large US-based population database highlights that survival of elderly patients has not improved relative to younger patients (2). This has resulted in growing scientific interest into the determinants of therapeutic success or failure for older patients. Several retrospective and a smaller number of prospective studies have been published in the last 5 years, aiming to optimize therapy in this age group.
Definition of ‘elderly’
The definition of ‘elderly’ remains a matter of intense debate for all hematological malignancies, and PCNSL is no exception. The main hurdle to reach universal consensus is the different life expectancy between different countries or regions, hence, patients who might be considered elderly in some countries/regions may be a candidate for intensive treatment in others. Historically, many clinical studies for PCNSL have defined ‘elderly’ patients as >60 years of age (8,9), although this cut-off has been empirically adopted. Another group of clinical trials focused on elderly patients, have included only patients >65 years (10-12) whereas the IELSG32 trial, evaluating strategies aimed at a ‘younger and fitter’ patient cohort has included patients aged 65–70 years with ECOG PS 0–2 at presentation (13).
Treatment with curative intent in PCNSL requires intensive induction chemotherapy (14) and clinical outcomes are highly reliant on post-induction consolidation using either high-dose chemotherapy followed by autologous stem cell transplantation (HDT-ASCT) or whole-brain radiotherapy (WBRT) (8,13,15-19). Treatment of PCNSL is unusual as clinical decision-making is frequently based on the perceived ‘fitness’ for intensive treatment and HDT-ASCT. Many studies have highlighted that fitness for HDT-ASCT cannot be defined strictly by an age threshold, as it encompasses additional factors such as comorbidities and tolerance to intensive induction chemotherapy (6,20,21). International consensus is urgently required to better define the criteria for ‘HDT-ASCT fitness’ that would help to harmonize the design of both prospective and retrospective studies.
Diagnostic particularities in PCNSL of the elderly
Timely diagnosis of PCNSL is paramount for all patients. Delays in initiating treatment are more common in elderly patients, potentially leading to inferior outcomes (5,22,23). Stereotactic biopsy to obtain a histological diagnosis remains the standard of care for PCNSL and older age should not obviate the need to obtain a histological diagnosis, whenever possible (24). The diagnostic pathway has been defined in a prior chapter of this series and includes cerebrospinal fluid assessment, slit lamp examination to rule out vitreoretinal involvement and exclusion of disease outside the CNS (25), which is relatively more important for the elderly as this would drastically alter the therapeutic strategy and prognosis.
Initiation of corticosteroids should be carefully considered in the elderly and ideally avoided in patients prior to biopsy (26). Corticosteroids are a recognized cause of non-diagnostic biopsies that could ultimately lead to delays of therapy and decreased PS at baseline. If clinically indicated, steroids should be weaned as soon as feasible or reduced to the minimum dose to increase the yield of brain parenchymal biopsies. Up-to-date MRI imaging after steroid cessation may be necessary for stereotactic planning as significant reductions in tumor size can be expected for PCNSL (27). Clinical/radiological response to steroids is not exclusive to PCNSL, although it may increase the likelihood of diagnosis (26). Steroids could also result in significant medical complications in already frail individuals, including exacerbation of hyperglycemia in patients with pre-existing diabetes mellitus and an increased risk of bacterial, viral and opportunistic infections. Finally, elderly patients are more susceptible to adrenal insufficiency after corticosteroid withdrawal, hence, cautious weaning schedules should be favored after treatment is initiated (28,29).
Cognitive impairment is more prevalent in an ageing population; initial neurological manifestations of PCNSL might be misinterpreted as apparent worsening of these symptoms, resulting in further diagnostics delays. Also, cognitive impairment typically complicates management, older PCNSL patients have higher nursing burden and often require additional nursing support to prevent falls and manage intravenous access/catheters.
Role of geriatric assessment in elderly PCNSL
Geriatric assessment is in general, a process aimed to a holistic assessment of elderly patients, evaluating somatic, psychological, functional and social domains alongside patient frailty. Geriatric assessment has been evaluated in elderly patients with hematological malignancies, providing evidence that impairment of certain domains is associated with treatment-related toxicity, treatment interruption and mortality (30-33).
Comprehensive geriatric assessment (CGA) is possibly the most accurate tool for assessment of comorbidities and one of the most widely used (34). CGA has been specifically applied to elderly patients with DLBCL (30,31,35) and other non-Hodgkin lymphoma (NHL) (36), demonstrating that CGA-fit and CGA-unfit patients have differential clinical outcomes. The main caveat of CGA is the lack of standardized scoring and the heterogeneity of the studies that have evaluated its usefulness.
Despite the relative lack of standardization, CGA has been used to guide treatment intensity in the elderly population. Spina et al. conducted a prospective study including 100 patients >70 years of age with newly diagnosed DLBCL and modulated chemoimmunotherapy with R-CHOP according to CGA scores with good clinical outcomes (37). Similarly, Olivieri et al. conducted a trial tailoring R-CHOP intensity according to CGA in patients ≥65 years, achieving excellent results in the CGA-fit subgroup with a 3-year overall survival (OS) above 70% (38). Both studies show that outcomes of CGA-fit patients mirror those of the general DLBCL population and although outcomes are worse for CGA-unfit patients, tailoring chemotherapy was able to keep treatment-related mortality (TRM) relatively low, at 11% and 5%, respectively.
CGA is invariably time consuming, which is the primary hurdle for its routine applicability. Abbreviated geriatric assessment tools have been proposed, such as the G8 screening tool (39). G8 has been tested in one elderly PCNSL cohort with successful discrimination of outcomes, Farhi et al. retrospectively recorded three comorbidity scores, the Charlson comorbidity index (CCI), the Cumulative Illness Rating Scale for Geriatrics (CIRS-G) and the G8 in a cohort of 35 elderly PCNSL patients. An association between a high CIRS-G score and shorter progression-free survival (PFS) and OS was found in univariate analysis, but could not be confirmed in multivariate analysis (40).
CCI is an additional assessment tool that has been extensively demonstrated to associate with outcomes in NHL (34,41-43). In PCNSL, CCI has been used less often, but has been associated with increased risk of acute kidney injury during methotrexate (MTX) treatment in univariate analysis (44) and with OS in a National Cancer Database US-based study (45).
Overall, there is a growing body of evidence that CGA and other geriatric assessments are able to predict outcomes in PCNSL and might be used to optimize therapy, as outlined for other aggressive NHL. Further research on the applicability and utility of the geriatric scales is clearly warranted.
Role of specific comorbidities
The most problematic comorbidities in elderly patients with PCNSL are those that potentially impair the ability to metabolize and/or excrete MTX, and the required intravenous fluids, namely renal function and left ventricular ejection fraction (LVEF) (7,46-48). Although LVEF is unlikely to directly impact MTX clearance, it does indirectly influence renal perfusion and determines the ability to manage the large volumes of intravenous hydration and bicarbonate required for each MTX dose. Multi-drug prescriptions are common in elderly, drug interactions are yet an additional source of delayed MTX elimination, worsened renal function and/or LV impairment, judicious and continuous review of medications is also important for appropriate care of these age group.
Up to 46% of the elderly subjects in the US National Health and Nutrition Evaluation Survey (NHANES) were considered to have CKD (CrCl <60 mL/min) (49). Creatinine clearance is typically the parameter used to define the suitability of MTX treatment, albeit no clear consensus regarding the threshold of clearance exists (50). A CrCl below 50–60 mL/min is widely accepted as a cut-off value to consider at least dose reduction of MTX (51-54). Tailoring of MTX dose to avoid or minimize increased exposure to MTX is desirable, exceedingly high MTX AUC levels have been demonstrated to have a negative impact on PFS and OS in elderly PCNSL patients (55,56). The Cockroft-Gault formula is the most widely used in the context of MTX, however, this calculation is clearly affected by extreme ages and weight, in such cases, 24-hour creatinine clearance might prove useful to guide decisions about MTX in selected patients (56).
Third space fluid accumulation can also result in increased MTX toxicity, due to an increase in distribution volume that leads to delayed clearance of the drug after a single administration (57). Third space fluid accumulation is more likely to develop in the elderly and should prompt dose adjustments if identified beforehand. Also, additional precautions to avoid vomiting and/or diarrhea would be useful to ensure a stable intravascular volume throughout the MTX administration and elimination.
Nutritional and rehabilitation support to optimize patients’ physical condition are also important as it may mitigate treatment-induced side effects and improve treatment tolerance. Input of a multidisciplinary support team in clinical management are paramount and have been associated with improvements of patient outcome (58).
Treatment of PCNSL in the elderly
Combination chemotherapy regimens incorporating high-dose methotrexate (HD-MTX) (>3 g/m2) are considered the standard of care for newly diagnosed PCNSL in younger cohorts (15,48,59-62). However, established HD-MTX treatment protocols for younger patients only represent an alternative for a selected subgroup of elderly PCNSL patients (20,21).
Choice of optimal treatment and delivery of adequate dose intensity of cytostatic treatment remain the biggest challenges when treating elderly patients (20,40). Available evidence suggests that optimal induction treatment for these patients remains HD-MTX-based immuno-chemotherapy (1,7,12,20,21,40,63). Subsequent consolidation treatment comprises HDT-ASCT (64,65), non-myeloablative chemotherapy (66), or WBRT (13,67-69).
The goal of treatment in the elderly remains achievement of complete remission (CR) (70). Individualized approaches adapting treatment intensity to age and/or comorbidities remains a challenge, but is clearly a desirable strategy, despite the lack of specific evidence. Chronological age, on its own, should not be a barrier for delivery of HD-MTX if physiological fitness is deemed adequate. Furthermore, baseline PS alone should not preclude intensive induction therapy as this often improves following initial treatment, allowing for subsequent intensification. Assessment of pre-morbid PS is paramount when assessing patients at diagnosis, as it will clearly aid clinical decisions regarding intended treatment intensity.
In practical terms, elderly PCNSL patients may be delineated into three treatment fitness groups: (I) eligible for intensive combination immuno-chemotherapy incorporating HDT-ASCT; (II) ineligible for HDT-ASCT, but eligible for HD-MTX-based immuno-chemotherapy and (III) ineligible for HD-MTX-based therapy: palliative treatment (a minority of patients). A proposed first-line treatment algorithm for elderly PCNSL patients according to eligibility for these three different treatment approaches is outlined (Figure 1).
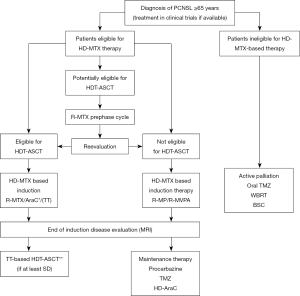
Intensive HD-MTX-based induction therapy
There are less data regarding intensive combination protocols in the treatment of older patients when compared to the body of evidence in younger patients receiving intensive HD-MTX approaches (13,17,61,71,72). Induction regimens in elderly patients typically employ 4–8 cycles of HD-MTX-based therapy, direct comparative data about the number of HD-MTX cycles administered are lacking (7).
MATRix combination regimen has demonstrated efficacy in elderly patients, the IELSG32 trial included patients up to the age of 70 years (with ECOG PS 0–2) and showed a 2-year PFS of 61% (95% CI: 55–67) (13,16). Remarkably, infective complications were more frequent in patients older than 60 years (13).
Prospective data has been echoed by retrospective ‘real world data’ studies. A recent international retrospective study of PCNSL patients, treated with the MATRix combination reproduced the toxicity and efficacy outcomes reported in the IELSG32 trial with response rates of 79% (95% CI: 71–85%), 2-year PFS of 56% (95% CI: 48–64%) and 2-year OS of 64% (95% CI: 56–72%) (compared to 86%, 61% and 69% of IELSG32 trial). TRM was comparable in both studies at approximately 6% (21). In this series, patients who would have not fulfilled the IELSG32 trial inclusion criteria (predominantly above 70 years or with impaired ECOG PS) were treated with lower dose intensity and experienced a significantly inferior outcome.
Another recently published retrospective study of 244 PCNSL patients >65 years treated in the UK, younger age was associated with improved response rates, PFS and OS, in those treated with more intensive chemoimmunotherapy strategies. Patients between 65 and 75 years experienced a 2-year PFS and OS of 45% and 52% compared to 38% and 32% for patients >75 years (20). OS of patients <75 years treated with intensive chemotherapy was comparable to a previously published dataset of older patients treated with MTX-based regimens (2-year OS of 49%). Interestingly, the TRM rate for patients >75 years (4.8%) was within the expected range for a PCNSL cohort. In this population-based study 79% of the consecutive diagnosed patients were able to receive HD-MTX-based regimens. When the subset of patients who achieved a MTX relative dose intensity of 0.75 was analyzed, survival outcomes compared favorably to published prospective studies. This is in line with other retrospective studies suggesting that MTX dose should be aimed at the maximal tolerated for the patients and that dose reductions in elderly subjects are very likely to impact treatment outcomes.
High rates of early treatment-related toxicity have been reported with HD-MTX regimens in the elderly, with 7% of the patients requiring intensive-care support and 40% requiring dose reductions in the first cycle of therapy (21). It is important to clarify that dose reductions during the first cycle did not seem to impact on PFS or OS suggesting that careful and early dose adjustments are feasible without compromising outcomes. This is a potentially useful strategy for elderly patients with poor PS at presentation, aiming to ramp up intensity in subsequent cycles if feasible. A less intensive pre-phase treatment (e.g., R-MTX) could reduce toxicity in early cycles and improve PS to achieve a high cumulative relative dose intensity in subsequent cycles. Evaluation of patients after pre-phase treatment would identify those able to tolerate further and more intensive cycles of treatment, as proposed in our algorithm (Figure 1). There is lack of specific evidence to recommend the optimal dose of the pre-phase R-MTX, dosing between 3 and 4 gr/m2 would be the author’s recommendation, mirroring the French (9) and German (10,73,74) elderly patient treatment protocols, provided creatinine clearance is above 50–60 mL/min.
In summary, there seems to be a clear subset of elderly patients with newly-diagnosed PCNSL who remain fit for an intensive and potentially curative approach to treatment. Diligent attention to supportive care and consideration of dose reductions, especially during cycle 1, are essential to mitigate against treatment-associated complications.
Dose-adapted HD-MTX combination regimens for the elderly
The main obstacle for delivery of treatment intensity in the elderly is the hematological toxicity, which in PCNSL regimens is largely driven by cytarabine (7). Substitution of cytarabine for oral alkylating agents is a widely used strategy to improve tolerability in the elderly. This has been evaluated in a recent meta-analysis that pooled individual patient data from 20 prospective and retrospective studies in PCNSL patients aged >60 years. HD-MTX-based therapy was associated with improved OS, although there was no discernible survival benefit to using intensive intravenous treatment protocols over HD-MTX combined with oral alkylating agents (6).
The PRIMAIN study has established the R-MP protocol [rituximab, procarbazine (PCZ), HD-MTX] now accepted as a treatment standard in Europe. PRIMAIN is the largest prospective study specifically designed for elderly PCNSL patients, it reported 1- and 2-year PFS rates of 46.3% (95% CI: 36–55%) and 37.3% (95% CI: 28–46%) and OS rates after 1 and 2 years of 56.7% (95% CI: 47–66%) and 47% (95% CI: 37–56%), respectively. R-MP was associated with a TRM rate of 8.4% and 81.3% of the patients who experienced ≥ grade 3adverse events. Most frequent severe toxicities were leukopenia (55.1%), infections (35.5%), and anemia (32.7%) (10,73).
The PRIMAIN study was preceded by a similar single-arm phase II study with the combination of HD-MTX, lomustine and PCZ, which served as a proof-of-principle trial, demonstrating efficacy of oral alkylating agents in combination with HD-MTX. This study reported an ORR of 44% and a 5-year OS of 33% (95% CI: 16–50%) (74).
Similarly, a randomized phase 2 study in patients >60 years (median age 72 years) compared HD-MTX, PCZ, vincristine and cytarabine (MPV-A) to HD-MTX and temozolomide (MT), without rituximab. No statistically significant difference between arms could be demonstrated, although outcomes appeared to favor MPV-A, with a 1-year PFS of 36% (95% CI: 22–50%) in both arms, median OS 31 months (95% CI: 12–35) for MPV-A and 14 months (95% CI: 8–24) for MT (9).
A subgroup analysis of the published G-PCNSL-SG-1 trial (75), focused on patients >70 years, has shown that careful dose-adjustment of HD-MTX was possible with the proposed dosing algorithm. Dose reductions were more frequent in elderly, although the number of patients >70 years was small and statistical power of the comparison is debatable. Toxicity was comparable to that of the younger cohort and response rates were also similar between the two groups, albeit survival outcomes were worse in the elderly, reflecting an overall lower cumulative dose intensity of MTX (12).
The modified Bonn protocols, which include reduced dose HD-MTX-based chemotherapy with temozolomide (TMZ) represents another alternative for a dose-adapted approach. Pels et al. reported use of a modified Bonn protocol in patients aged 65–75 years (median age 70 years) with estimated 2-year OS of 55.6% (95% CI: 35.3–71.8%) (76). These results are surprisingly similar to a younger cohort receiving a more intensive version of the same regimen (2-year OS 60.7%, 95% CI: 43.3–74.2%) (11). The rituximab-MTX-TMZ regimen has been evaluated in a retrospective study, showing benefit of addition of Rituximab to the MTX-TMZ combination as induction therapy, followed by HDT-ASCT (3%) or WBRT (32%) consolidation therapy (77).
Optimization of HD-MTX protocols for elderly patients who are not HDT-ASCT candidates continues to be an area of intensive research, the authors believe HD-MTX combination regimens should be offered to everyone able to tolerate it as it clearly extends survival compared to best supportive care and there is a sizeable proportion of patients who achieve long-term remission (9,10,20,40,59,74). Clinical trials, wherever available, should also be strongly considered.
Consolidation therapy
Consolidation treatment in PCNSL is typically offered to patients who achieve at least a partial remission with induction chemo-immunotherapy. One of the paradigms of PCNSL treatment is the high influence of the consolidation treatment in the long-term outcomes. Highly CNS penetrating agents in the context of a HDT-ASCT seem to increase the probability of disease eradication (14,18,71,78). Akin to induction treatment, the level of evidence for consolidation in elderly patients is considerably less when compared to younger cohorts.
In the recent large UK retrospective study of patients >65 years, the small subset of patients who received consolidation treatment (22%) showed 2-year PFS of 62% and OS of 73%, roughly comparable to benchmark trials in younger patients (8,9,15,20,72,78). These patients had mostly HDT-ASCT (14%) and only a minority WBRT (8%). Most patients proceeding to consolidation received induction therapy with MATRix or HD-MTX/AraC combinations.
Consolidation with HDT-ASCT
HDT-ASCT has become a widely accepted consolidation approach, reserved for elderly PCNSL patients who are able to tolerate aggressive systemic chemotherapy (64,65). Nevertheless, a retrospective analysis from the French LOC reported that only 2% of patients >60 years received HDT-ASCT consolidation during the period 2011–2016 (5), suggesting HDT-ASCT approach in the elderly is highly dependent on the treating institution and the local practice.
Various single arm (17,55,71,79-81) and two randomized trials (8,13,16,82) provide a robust body of evidence for the use of thiotepa-based HDT-ASCT protocols in PCNSL patients <65–70 years, with 3–5 year OS rates between 69% (13) and 87% (17). To date, the prospective bicentric trial MARiTA is the only trial which specifically investigated HDT-ASCT in elderly (>65 years) PCNSL patients, MARiTA reported 2-year PFS and OS rates of 92.9% (95% CI: 80–100%) and 92.3% (95% CI: 78–100%), without TRM (83). As expected, grade >3 hematological toxicity was more frequent in patients treated with the more intensive HDT-ASCT approach, but infective complications were similar to those reported in the PRIMAIN study (10,73).
Several retrospective studies have also addressed this issue, one of them investigated HDT-ASCT in eligible elderly PCNSL patients in first (n=15) and subsequent therapy lines (n=37), with an overall response rate of 79% (95% CI: 71–85%) and a 2-year PFS rate of 56% (95% CI: 48–64%). Noticeably, 2-year PFS and OS rates of those patients who received HDT-ASCT as first-line treatment were 80% (64).
Thiotepa continues to be the preferred agent for HDT-ASCT consolidation in the setting of HD-MTX induction regimens. Interestingly, a Japanese series featuring a group who received thiotepa-based HDT-ASCT compared to a non-thiotepa based conditioning, has shown a HR of 0.42 (95% CI: 0.19–0.95) for survival when thiotepa was used in the conditioning regimen (84). The two groups arose because of the lack of availability of thiotepa in Japan after 2011, providing a unique opportunity to compare both conditionings without the bias of the transplanting centers choice of conditioning, although the impact of the confounding variables within the different “eras” was acknowledged.
Albeit evidence has become available over the last years, randomized controlled trials of HDT-ASCT consolidation in older patients, confirming the preliminary finding of the various retrospective studies and the MARiTA trial, are lacking. The MARTA trial is an ongoing phase II that will investigate further HDT-ASCT in the elderly patient population (DRKS00011932) (65). To determine the precise role of HDT-ASCT in the elderly population, phase III trials comparing an age-adapted thiotepa-containing HDT-ASCT approach with non-intensive approaches (e.g., R-MP protocol) are needed.
Consolidation with WBRT
Many elderly patients will be unfit for ASCT despite tolerating HD-MTX based therapy. Many studies have evaluated WBRT consolidation in younger cohorts, or age-unselected cohorts. In general, results suggest that WBRT is an effective consolidation strategy and it may offer modest PFS and OS improvements at the price of neurotoxicity.
WBRT has been used as consolidation in first CR after chemoimmunotherapy (16,17,19,67,79), which continues to be its main use despite the neurocognitive risks associated with it. Most protocols empirically use a WBRT dose of 36–45 Gy although it should be emphasized that the optimal total dose and role of a tumor bed ‘boost’ remains uncertain (85), particularly in the era of more intensive remission induction protocols.
WBRT role in the era of modern chemoimmunotherapy treatment algorithms and the alternative of HDT-ASCT consolidation is a matter of international debate. There are three randomized trials, one addressing the question if WBRT can be omitted in patients in first CR (75,86) and two trials comparing WBRT with HDT-ASCT (8,16), none of which have specifically addressed this question in the elderly, only two trials have included elderly patients ≤70 years (16,75).
The IELSG32 trial reported 2-year OS of 80% (95% CI: 70–90%) vs. 69% (95% CI: 59–79%) between WBRT and HDT-ASCT consolidation, respectively (16). In a randomized phase 3 trial comparing standard WBRT (45 Gy) with no further treatment in patients achieving CR following MTX-based chemotherapy (75), the primary endpoint was not met, improved PFS was reported in the WBRT group but it did not translate into an OS advantage. More importantly, clinically assessed neurotoxicity doubled in the WBRT arm alongside neuroradiology assessed neurotoxicity.
The cognitive sequelae of WBRT have been evaluated by Correa et al. (87). Employing a wide battery of cognitive tests, assessment focused on three main areas (attention/executive functions, verbal memory, and graphmotor speed). Of these areas, attention/executive function and verbal memory were significantly impaired in the group that received WBRT consolidation, making HDT-ASCT a preferable strategy from the perspective of cognitive function preservation.
In line with these findings, a systematic meta-analysis found no clear overall benefit in terms of quality-adjusted life years for WBRT in first remission in patients >60 years (88). Elderly patients appeared to have an increased risk for delayed neurotoxicity and declined neurocognitive function after WBRT (89). The dosage of WBRT has also been a matter of research to improve long-term toxicity, trials have investigated hyper-fractionated or lower radiation doses to minimize neurotoxicity (16,68,87,90,91). Use of reduced dose radiotherapy (23.4 Gy) as consolidation for patients in CR after rituximab, MTX, PCZ, and vincristine (R-MPV) induction has been explored by Morris et al. in a non-randomized phase 2 study. In this subset of patients, survival and neurocognitive outcomes were encouraging, but the number of evaluable patients was small (12 of 31 who received WBRT and only 3 patients ≥60 years of age) (19).
A recently reported retrospective study comparing outcome and neurotoxicity between patients receiving consolidation with reduced-dose WBRT (≤23.4 Gy) and standard-dose WBRT (>23.4 Gy) showed that the former approach reduces neurotoxicity especially in patients <60 years with no difference in outcome. Of note, neurotoxicity was observed in patients >60 years also in the reduced-dose WBRT arm but still at a much lower rate than in the standard-dose WBRT arm (85). In another small study with a total of 18 patients, 9 patients receiving reduced-dose (23.4 Gy) WBRT in CR, had a higher relapse rate when compared to 9 patients who received WBRT at a dose of 45 Gy in PR (92).
The French LOC network retrospectively evaluated treatment and outcome of 1,002 PCNSL patients showed that WBRT consolidation was used in only 2% of patients >60 years (5). Although the number of elderly patients consolidated with WBRT was too small to draw firm conclusions, the outcomes of those patients consolidated with WBRT improved when compared to palliative treatment. WBRT consolidation was also infrequent in a large retrospective study from the UK (8% of consolidated patients) (20), both studies reflecting an evolution of practice favoring chemotherapy-based consolidation in elderly patients. Randomized studies are needed to validate the dose of WBRT at which the best balance of efficacy and long-term consequences is achieved for elderly patients.
Consolidation with conventional chemotherapy
TMZ maintenance has been adopted in PCNSL from the experience from glioblastoma and other primary CNS malignancies (93-97), it offers a low-toxic metronomic treatment with good blood-brain-barrier penetration (98) and demonstrated activity in PCNSL (99-101). Specifically on this matter, the previously mentioned phase II study from Nordic Lymphoma Group (11) offering TMZ maintenance after HD-MTX induction, showed 80.8% ORR after induction and 2-year OS of 55.6% after TMZ maintenance. Although a small cohort (n=26), the results of the TMZ maintenance arm were promising and strongly suggest TMZ is an alternative for elderly patients after induction therapy.
The RTOG 0227 phase I–II trial reported 2-year PFS and OS of 63.6% and 80.8% with a HD-MTX and TMZ based induction, hyperfractionated WBRT and TMZ maintenance. Although only 38% of the cohort exceeded 60 years of age and the independent effect of TMZ is impossible to dissect, this trial provides good evidence of HD-MTX/TMZ activity in PCNSL (68).
Another small series (n=10) using TMZ single agent as maintenance after R-MPV in patients ≥60 years with promising outcomes, showing 2-year PFS of 67% and 2-year OS of 88% (102).
There are two main issues with TMZ treatment in this setting. Firstly, oral treatment assumes ability to swallow tablets and comply with complex/multi-drug prescriptions, conditions that are frequently not met in elderly patients. Secondly, although considered to have a low toxicity profile, TMZ can cause significant hematological toxicity in 5–10% of patients (103). Thrombocytopenia is of particular relevance, requiring continued monitoring and dose adjustments.
PCZ is an alternative alkylating agent that has been used for consolidation/maintenance in elderly PCNSL patients. The evidence for PCZ maintenance comes mainly from the PRIMAIN trial, a single-arm phase II study for patients >65 years, using an attenuated induction followed by PCZ maintenance for 6 months given to responders. Interestingly, CR was 35.5% after induction treatment and best CR rate was 42%, implying PCZ maintenance contributed to an improvement of initial response during the maintenance phase (10). There is no comparative data between PCZ and TMZ for consolidation in the elder subgroup, the FIORELLA trial (NCT03495960) is aiming to address this question randomizing PCZ or lenalidomide after R-MP induction regimen.
Single-agent cytarabine maintenance has also been investigated after HD-MTX-based induction. The LOC network study for elderly PCNSL patients ≥60 years showed a 55% CR rate after cytarabine maintenance (3 g/m2 on day 1 and 2 of each cycle), with median PFS of 10 months (95% CI: 6–13) and median OS of 28 months (95% CI: 7–48) (104). CR rate after induction was 24%, suggesting that maintenance with cytarabine was able to improve CR rate with 1 toxic death (2%) recorded during the maintenance phase.
Role of palliative and best supportive care
There is still a minority of patients, that are considered unfit for MTX-based therapy from the outset. Data is scarce and the level of evidence poor to guide treatment decisions in this context. The proportion of patients (21%) who were reported to receive palliative care in a UK retrospective cohort involving 14 centers (20) was higher than in previously published single-center series of patients >65 years (13% receiving active palliation including radiotherapy) (59).
Life expectancy, PS and quality of life (QoL) are important factors to take into consideration when discussing treatment options. WBRT, corticosteroids, oral chemotherapy, with or without concomitant rituximab, are common approaches in this setting (58). Best supportive care is, unfortunately, the only viable option in some cases.
As for oral chemotherapy, a small retrospective study of single agent TMZ in elderly patients (n=19) showed a CR rate of 47%, with prolonged responses (>12 months) in 29.4%, median PFS 5 months, and median OS 21 months (105). In this context, the methylation status of MGMT promoter has been proposed as a baseline predictor of response to TMZ (101). Numbers remain too small to draw definitive conclusions but the concept of individualized therapy in the context of HD-MTX unfit patients remains highly promising.
Outcomes of relapsed PCNSL in the elderly is dismal, the majority of patients will be unfit for salvage chemotherapy (106). A French LOC network study including 256 relapsed patients showed survival of 0.6 (range, 0–5) months for those who did not receive salvage and 8.4 (range, 0–29) months for those who received second line treatment (107).
Novel therapies are urgently needed for this group of patients. Recent evidence supports a role for BTK inhibitors in relapsed PCNSL with high rates of response (108), albeit relatively short PFS in elderly patients (109-112). Lenalidomide-rituximab combinations have also yielded promising results with an ORR of 67% and PFS of 8.1 months (95% CI: 4.2–NR) in phase 2 studies (113). Long-term remission is unlikely to be achieved with any of these novel agents in monotherapy, thus its combination with chemotherapeutic agents merits to be investigated further.
Palliative WBRT
WBRT could also represent a palliative therapeutic alternative for newly diagnosed patients, accepting the high risk of neuropsychological sequelae in patients who are otherwise unfit for chemotherapy (114). In general, lower doses and shorter treatment durations (20–30 Gy in 1.8–4 Gy fractions) could be a more pragmatic approach for the palliation strategy.
WBRT with methylprednisolone has been evaluated in a small cohort of 19 patients ≥70 years. Patients who received methylprednisolone initially had a median EFS and OS of 11.7 and 12.1 months, respectively (115). Several retrospective studies have addressed this question, a multicenter retrospective study of PCNSL treated with upfront WBRT shows 1-year OS of 41% and a median survival of 7 (range, 1–64) months (116). A single-center retrospective study has shown an OS of 8 months (95% CI: 3–13) after upfront WBRT, compared to 3 months for best supportive care, suggesting WBRT could have a role in active palliation of patients unfit for intensive chemotherapy (117). Another single-center retrospective study in patients ≥60 years, showed WBRT alone (40 Gy + 20 Gy boost) gave a median survival of only 7.6 months (114).
Although no specific studies in the elderly have been published, a number of guidelines and reviews outline WBRT use in this setting (58,118). Further information about the value of dose-attenuated WBRT will be available from the arm B of the FIORELLA trial [patients not eligible for HD-MTX: WBRT 23.4 Gy + TMZ + rituximab followed by TMZ maintenance (NCT03495960)].
Post-treatment follow up
Post-treatment follow up has two core aspects, imaging follow-up and post-treatment neuropsychological assessments.
Prospective data are lacking, hence most guidelines have issued recommendations based on expert opinion. Current guidelines do not differ in elderly patients and consider follow-up imaging by contrast-enhanced MRI every 3 months for 2 years and less frequently until 10 years (58,118). Available evidence suggest no clear benefit for surveillance imaging as only 6–25% of all relapses are truly asymptomatic and hence likely to be detected on follow-up imaging (107,119,120). Routine imaging post-treatment may be useful in the surveillance of intracranial sequelae of treatment, where appropriate (e.g., hydrocephalus, white matter atrophy).
Neuropsychological assessments
Cognitive impairment is a frequent complication of PCNSL treatment with inevitable heterogeneity in neuropsychological deficits due to the variable tumor locations (121). WBRT is a recognized risk for delayed neurotoxicity but cognitive impairment has been documented after HD-MTX treatment and also after ASCT (91).
In a review of 17 studies, cognitive impairment was found >24 months after treatment in most PCNSL patients treated with WBRT plus chemotherapy whereas patients treated with chemotherapy alone had either stable or improved cognitive performance (90). In the longest observational study of 80 PCNSL survivors in CR, patients receiving WBRT had lower mean scores in attention/executive function, motor skills and overall neuropsychological composite score compared with those treated without WBRT (91). Treatment-related cognitive morbidity is also associated with a lower QoL and poor prognosis (88,91).
Although standardization is challenging, neuropsychological assessment should be an important part of follow up examinations. For older patients in particular many potential confounding factors may exist, making the interpretation of neurocognitive results a considerable challenge and requiring specialized personnel. In the absence of neuropsychology support, baseline and serial mini mental state examination has been suggested as a minimum requirement (24).
The optimal neurocognitive test battery and interval of assessment for PCNSL patients remains unclear and discussed further in another chapter of this series. Correa et al. proposed a minimum core battery for the assessment of neuropsychological functions and QoL in PCNSL patients (90). The whole battery can be completed in less than 40 minutes and has been successfully adapted to the most recently designed prospective trials, data are awaited (87).
Conclusions
Incidence of PNCSL in the elderly is increasing in parallel to other hematological malignancies. Diagnosis and treatment of elderly PCNSL poses significant challenges to clinicians, these patients are more resource intensive, more likely to experience complications during treatment. Also, the overall amount of evidence available specifically for this age group is scant.
Diagnostic procedures and treatment should be tailored carefully to age and comorbidities, for this, geriatric assessments are increasingly being recognized as valuable and reproducible tools. Elderly patients will have a greater impact of the disease on their PS, hence decisions to offer/negate treatment should also take into consideration pre-morbid PS.
HD-MTX-based therapy continues to be the best available treatment option and has been demonstrated to be feasible in at least three-quarters of the elderly population. HDT-ASCT remains an option for a minority, but should be considered in ‘fit’ patients as outcomes compare to the younger population.
The main areas of unmet need in the elderly PCNSL population are the patients deemed unfit to HD-MTX and the relapsed population, both with dismal prognosis. Well-designed trials are warranted, which incorporate age-adapted therapies and combinations of novel agents with moderate toxicity profiles.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Andrés J. M. Ferreri, Maurilio Ponzoni) for the series “Central Nervous System Lymphomas” published in Annals of Lymphoma. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aol-20-30). The series “Central Nervous System Lymphomas” was commissioned by the editorial office without any funding or sponsorship. KC reports Consulting/Advisory Role for Roche, Takeda, Celgene, Atara, Gilead, KITE and Janssen; Speakers’ Bureau of Roche, Takeda, KITE and Gilead; Conferences/travel support from Roche, Takeda, KITE and Janssen. ES reports Speakers’ honoraries and research funding from Riemser Pharma GmbH. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all the aspects of this manuscript in ensuring that questions related to the accuracy and integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegal T, Bairey O. Primary CNS lymphoma in the elderly: the challenge. Acta Haematol 2019;141:138-45. [Crossref] [PubMed]
- Mendez JS, Ostrom QT, Gittleman H, et al. The elderly left behind-changes in survival trends of primary central nervous system lymphoma over the past 4 decades. Neuro Oncol 2018;20:687-94. [Crossref] [PubMed]
- Gittleman H, Boscia A, Ostrom QT, et al. Survivorship in adults with malignant brain and other central nervous system tumor from 2000-2014. Neuro Oncol. 2018;20:vii6-16. [Crossref] [PubMed]
- Furst T, Hoffman H, Chin LS. All-cause and tumor-specific mortality trends in elderly primary central nervous system lymphoma (PCNSL) patients: a surveillance, epidemiology, and end results (SEER) analysis. J Neurosurg Sci 2019; Epub ahead of print. [Crossref] [PubMed]
- Houillier C, Soussain C, Ghesquieres H, et al. Management and outcome of primary CNS lymphoma in the modern era: an LOC network study. Neurology 2020;94:e1027-39. [Crossref] [PubMed]
- Kasenda B, Ferreri AJ, Marturano E, et al. First-line treatment and outcome of elderly patients with primary central nervous system lymphoma (PCNSL)--a systematic review and individual patient data meta-analysis. Ann Oncol 2015;26:1305-13. [Crossref] [PubMed]
- Roth P, Hoang-Xuan K. Challenges in the treatment of elderly patients with primary central nervous system lymphoma. Curr Opin Neurol 2014;27:697-701. [Crossref] [PubMed]
- Houillier C, Taillandier L, Dureau S, et al. Radiotherapy or autologous stem-cell transplantation for primary CNS lymphoma in patients 60 years of age and younger: results of the intergroup ANOCEF-GOELAMS randomized phase II PRECIS study. J Clin Oncol 2019;37:823-33. [Crossref] [PubMed]
- Omuro A, Chinot O, Taillandier L, et al. Methotrexate and temozolomide versus methotrexate, procarbazine, vincristine, and cytarabine for primary CNS lymphoma in an elderly population: an intergroup ANOCEF-GOELAMS randomised phase 2 trial. Lancet Haematol 2015;2:e251-9. [Crossref] [PubMed]
- Fritsch K, Kasenda B, Schorb E, et al. High-dose methotrexate-based immuno-chemotherapy for elderly primary CNS lymphoma patients (PRIMAIN study). Leukemia 2017;31:846-52. [Crossref] [PubMed]
- Pulczynski EJ, Kuittinen O, Erlanson M, et al. Successful change of treatment strategy in elderly patients with primary central nervous system lymphoma by de-escalating induction and introducing temozolomide maintenance: results from a phase II study by the Nordic Lymphoma Group. Haematologica 2015;100:534-40. [Crossref] [PubMed]
- Roth P, Martus P, Kiewe P, et al. Outcome of elderly patients with primary CNS lymphoma in the G-PCNSL-SG-1 trial. Neurology 2012;79:890-6. [Crossref] [PubMed]
- Ferreri AJ, Cwynarski K, Pulczynski E, et al. Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol 2016;3:e217-27. [Crossref] [PubMed]
- Ferreri AJ. How I treat primary CNS lymphoma. Blood 2011;118:510-22. [Crossref] [PubMed]
- Abrey LE, Moskowitz CH, Mason WP, et al. Intensive methotrexate and cytarabine followed by high-dose chemotherapy with autologous stem-cell rescue in patients with newly diagnosed primary CNS lymphoma: an intent-to-treat analysis. J Clin Oncol 2003;21:4151-6. [Crossref] [PubMed]
- Ferreri AJM, Cwynarski K, Pulczynski E, et al. Whole-brain radiotherapy or autologous stem-cell transplantation as consolidation strategies after high-dose methotrexate-based chemoimmunotherapy in patients with primary CNS lymphoma: results of the second randomisation of the International Extranodal Lymphoma Study Group-32 phase 2 trial. Lancet Haematol 2017;4:e510-23. [Crossref] [PubMed]
- Illerhaus G, Marks R, Ihorst G, et al. High-dose chemotherapy with autologous stem-cell transplantation and hyperfractionated radiotherapy as first-line treatment of primary CNS lymphoma. J Clin Oncol 2006;24:3865-70. [Crossref] [PubMed]
- Kasenda B, Schorb E, Fritsch K, et al. Prognosis after high-dose chemotherapy followed by autologous stem-cell transplantation as first-line treatment in primary CNS lymphoma--a long-term follow-up study. Ann Oncol 2012;23:2670-5. [Crossref] [PubMed]
- Morris PG, Correa DD, Yahalom J, et al. Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: final results and long-term outcome. J Clin Oncol 2013;31:3971-9. [Crossref] [PubMed]
- Martinez-Calle N, Poynton E, Alchawaf A, et al. Outcomes of older patients with primary central nervous system lymphoma treated in routine clinical practice in the UK: methotrexate dose intensity correlates with response and survival. Br J Haematol 2020;190:394-404. [Crossref] [PubMed]
- Schorb E, Fox CP, Kasenda B, et al. Induction therapy with the MATRix regimen in patients with newly diagnosed primary diffuse large B-cell lymphoma of the central nervous system - an international study of feasibility and efficacy in routine clinical practice. Br J Haematol 2020;189:879-87. [Crossref] [PubMed]
- Cerqua R, Balestrini S, Perozzi C, et al. Diagnostic delay and prognosis in primary central nervous system lymphoma compared with glioblastoma multiforme. Neurol Sci 2016;37:23-9. [Crossref] [PubMed]
- Morell AA, Shah AH, Cavallo C, et al. Diagnosis of primary central nervous system lymphoma: a systematic review of the utility of CSF screening and the role of early brain biopsy. Neurooncol Pract 2019;6:415-23. [Crossref] [PubMed]
- Abrey LE, Batchelor TT, Ferreri AJ, et al. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol 2005;23:5034-43. [Crossref] [PubMed]
- Mohile NA, Deangelis LM, Abrey LE. The utility of body FDG PET in staging primary central nervous system lymphoma. Neuro Oncol 2008;10:223-8. [Crossref] [PubMed]
- Gessler F, Bernstock JD, Behmanesh B, et al. The impact of early corticosteroid pretreatment before initiation of chemotherapy in patients with primary central nervous system lymphoma. Neurosurgery 2019;85:264-72. [Crossref] [PubMed]
- Manoj N, Arivazhagan A, Mahadevan A, et al. Central nervous system lymphoma: patterns of incidence in Indian population and effect of steroids on stereotactic biopsy yield. Neurol India 2014;62:19-25. [Crossref] [PubMed]
- Laugesen K, Petersen I, Sorensen HT, et al. Clinical indicators of adrenal insufficiency following discontinuation of oral glucocorticoid therapy: a Danish population-based self-controlled case series analysis. PLoS One 2019;14:e0212259 [Crossref] [PubMed]
- Dinsen S, Baslund B, Klose M, et al. Why glucocorticoid withdrawal may sometimes be as dangerous as the treatment itself. Eur J Intern Med 2013;24:714-20. [Crossref] [PubMed]
- Ong DM, Ashby M, Grigg A, et al. Comprehensive geriatric assessment is useful in an elderly Australian population with diffuse large B-cell lymphoma receiving rituximab-chemotherapy combinations. Br J Haematol 2019;187:73-81. [Crossref] [PubMed]
- Ribi K, Rondeau S, Hitz F, et al. Cancer-specific geriatric assessment and quality of life: important factors in caring for older patients with aggressive B-cell lymphoma. Support Care Cancer 2017;25:2833-42. [Crossref] [PubMed]
- Scheepers ERM, Vondeling AM, Thielen N, et al. Geriatric assessment in older patients with a hematologic malignancy: a systematic review. Haematologica 2020;105:1484-93. [Crossref] [PubMed]
- Tucci A, Martelli M, Rigacci L, et al. Comprehensive geriatric assessment is an essential tool to support treatment decisions in elderly patients with diffuse large B-cell lymphoma: a prospective multicenter evaluation in 173 patients by the Lymphoma Italian Foundation (FIL). Leuk Lymphoma 2015;56:921-6. [Crossref] [PubMed]
- Terret C, Albrand G, Rainfray M, et al. Impact of comorbidities on the treatment of non-Hodgkin's lymphoma: a systematic review. Expert Rev Hematol 2015;8:329-41. [Crossref] [PubMed]
- Merli F, Luminari S, Rossi G, et al. Outcome of frail elderly patients with diffuse large B-cell lymphoma prospectively identified by Comprehensive Geriatric Assessment: results from a study of the Fondazione Italiana Linfomi. Leuk Lymphoma 2014;55:38-43. [Crossref] [PubMed]
- Winkelmann N, Petersen I, Kiehntopf M, et al. Results of comprehensive geriatric assessment effect survival in patients with malignant lymphoma. J Cancer Res Clin Oncol 2011;137:733-8. [Crossref] [PubMed]
- Spina M, Balzarotti M, Uziel L, et al. Modulated chemotherapy according to modified comprehensive geriatric assessment in 100 consecutive elderly patients with diffuse large B-cell lymphoma. Oncologist 2012;17:838-46. [Crossref] [PubMed]
- Olivieri A, Gini G, Bocci C, et al. Tailored therapy in an unselected population of 91 elderly patients with DLBCL prospectively evaluated using a simplified CGA. Oncologist 2012;17:663-72. [Crossref] [PubMed]
- Hamaker ME, Prins MC, Stauder R. The relevance of a geriatric assessment for elderly patients with a haematological malignancy--a systematic review. Leuk Res 2014;38:275-83. [Crossref] [PubMed]
- Farhi J, Laribi K, Orvain C, et al. Impact of front line relative dose intensity for methotrexate and comorbidities in immunocompetent elderly patients with primary central nervous system lymphoma. Ann Hematol 2018;97:2391-401. [Crossref] [PubMed]
- Wästerlid T, Mohammadi M, Smedby KE, et al. Impact of comorbidity on disease characteristics, treatment intent and outcome in diffuse large B-cell lymphoma: a Swedish lymphoma register study. J Intern Med 2019;285:455-68. [Crossref] [PubMed]
- Wieringa A, Boslooper K, Hoogendoorn M, et al. Comorbidity is an independent prognostic factor in patients with advanced-stage diffuse large B-cell lymphoma treated with R-CHOP: a population-based cohort study. Br J Haematol 2014;165:489-96. [Crossref] [PubMed]
- Saygin C, Jia X, Hill B, et al. Impact of comorbidities on outcomes of elderly patients with diffuse large B-cell lymphoma. Am J Hematol 2017;92:989-96. [Crossref] [PubMed]
- Amitai I, Rozovski U, El-Saleh R, et al. Risk factors for high-dose methotrexate associated acute kidney injury in patients with hematological malignancies. Hematol Oncol 2020;38:584-8. [Crossref] [PubMed]
- Fallah J, Qunaj L, Olszewski AJ. Therapy and outcomes of primary central nervous system lymphoma in the United States: analysis of the National Cancer Database. Blood Adv 2016;1:112-21. [Crossref] [PubMed]
- Howard SC, McCormick J, Pui CH, et al. Preventing and managing toxicities of high-dose methotrexate. Oncologist 2016;21:1471-82. [Crossref] [PubMed]
- Welch MR, Omuro A, Deangelis LM. Outcomes of the oldest patients with primary CNS lymphoma treated at Memorial Sloan-Kettering Cancer Center. Neuro Oncol 2012;14:1304-11. [Crossref] [PubMed]
- Zhu JJ, Gerstner ER, Engler DA, et al. High-dose methotrexate for elderly patients with primary CNS lymphoma. Neuro Oncol 2009;11:211-5. [Crossref] [PubMed]
- Stevens LA, Viswanathan G, Weiner DE. Chronic kidney disease and end-stage renal disease in the elderly population: current prevalence, future projections, and clinical significance. Adv Chronic Kidney Dis 2010;17:293-301. [Crossref] [PubMed]
- Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. Oncologist 2006;11:694-703. [Crossref] [PubMed]
- Kintzel PE, Dorr RT. Anticancer drug renal toxicity and elimination: dosing guidelines for altered renal function. Cancer Treat Rev 1995;21:33-64. [Crossref] [PubMed]
- Bennett WM. Drug prescribing in renal failure: dosing guidelines for adults and children. 5th ed. Philadelphia: American College of Physicians, 2007.
- CancerCareOntario. Methotrexate. CCO Formulary for MTX. Adjustments by CrCl. (cited 2020 Sep 05). Available online: https://www.cancercareontario.ca/en/drugformulary/drugs/monograph/44166
- Aronoff GR, Bennett WM, Berns JS, et al. Drug prescribing in renal failure. 5th ed. Philadelphia: American College of Physicians, 2007.
- Kasenda B, Rehberg M, Thurmann P, et al. The prognostic value of serum methotrexate area under curve in elderly primary CNS lymphoma patients. Ann Hematol 2012;91:1257-64. [Crossref] [PubMed]
- Ferreri AJ, Guerra E, Regazzi M, et al. Area under the curve of methotrexate and creatinine clearance are outcome-determining factors in primary CNS lymphomas. Br J Cancer 2004;90:353-8. [Crossref] [PubMed]
- Li J, Gwilt P. The effect of malignant effusions on methotrexate disposition. Cancer Chemother Pharmacol 2002;50:373-82. [Crossref] [PubMed]
- Fox CP, Phillips EH, Smith J, et al. Guidelines for the diagnosis and management of primary central nervous system diffuse large B-cell lymphoma. Br J Haematol 2019;184:348-63. [Crossref] [PubMed]
- Ney DE, Reiner AS, Panageas KS, et al. Characteristics and outcomes of elderly patients with primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center experience. Cancer 2010;116:4605-12. [Crossref] [PubMed]
- Zeremski V, Koehler M, Fischer T, et al. Characteristics and outcome of patients with primary CNS lymphoma in a "real-life" setting compared to a clinical trial. Ann Hematol 2016;95:793-9. [Crossref] [PubMed]
- Ferreri AJ, Reni M, Foppoli M, et al. High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: a randomised phase 2 trial. Lancet 2009;374:1512-20. [Crossref] [PubMed]
- Ferreri AJ, Reni M, Pasini F, et al. A multicenter study of treatment of primary CNS lymphoma. Neurology 2002;58:1513-20. [Crossref] [PubMed]
- Olivier G, Clavert A, Lacotte-Thierry L, et al. A phase 1 dose escalation study of idarubicin combined with methotrexate, vindesine, and prednisolone for untreated elderly patients with primary central nervous system lymphoma. The GOELAMS LCP 99 trial. Am J Hematol 2014;89:1024-9. [Crossref] [PubMed]
- Schorb E, Fox CP, Fritsch K, et al. High-dose thiotepa-based chemotherapy with autologous stem cell support in elderly patients with primary central nervous system lymphoma: a European retrospective study. Bone Marrow Transplant 2017;52:1113-9. [Crossref] [PubMed]
- Schorb E, Finke J, Ihorst G, et al. Age-adjusted high-dose chemotherapy and autologous stem cell transplant in elderly and fit primary CNS lymphoma patients. BMC Cancer 2019;19:287. [Crossref] [PubMed]
- Schorb E, Finke J, Ferreri AJ, et al. High-dose chemotherapy and autologous stem cell transplant compared with conventional chemotherapy for consolidation in newly diagnosed primary CNS lymphoma--a randomized phase III trial (MATRix). BMC Cancer 2016;16:282. [Crossref] [PubMed]
- DeAngelis LM, Seiferheld W, Schold SC, et al. Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: Radiation Therapy Oncology Group Study 93-10. J Clin Oncol 2002;20:4643-8. [Crossref] [PubMed]
- Glass J, Won M, Schultz CJ, et al. Phase I and II study of induction chemotherapy with methotrexate, rituximab, and temozolomide, followed by whole-brain radiotherapy and postirradiation temozolomide for primary CNS lymphoma: NRG oncology RTOG 0227. J Clin Oncol 2016;34:1620-5. [Crossref] [PubMed]
- Hoang-Xuan K, Bessell E, Bromberg J, et al. Diagnosis and treatment of primary CNS lymphoma in immunocompetent patients: guidelines from the European Association for Neuro-Oncology. Lancet Oncol 2015;16:e322-32. [Crossref] [PubMed]
- Pels H, Juergens A, Schirgens I, et al. Early complete response during chemotherapy predicts favorable outcome in patients with primary CNS lymphoma. Neuro Oncol 2010;12:720-4. [Crossref] [PubMed]
- Illerhaus G, Kasenda B, Ihorst G, et al. High-dose chemotherapy with autologous haemopoietic stem cell transplantation for newly diagnosed primary CNS lymphoma: a prospective, single-arm, phase 2 trial. Lancet Haematol 2016;3:e388-97. [Crossref] [PubMed]
- Rubenstein JL, Hsi ED, Johnson JL, et al. Intensive chemotherapy and immunotherapy in patients with newly diagnosed primary CNS lymphoma: CALGB 50202 (Alliance 50202). J Clin Oncol 2013;31:3061-8. [Crossref] [PubMed]
- Fritsch K, Kasenda B, Hader C, et al. Immunochemotherapy with rituximab, methotrexate, procarbazine, and lomustine for primary CNS lymphoma (PCNSL) in the elderly. Ann Oncol 2011;22:2080-5. [Crossref] [PubMed]
- Illerhaus G, Marks R, Muller F, et al. High-dose methotrexate combined with procarbazine and CCNU for primary CNS lymphoma in the elderly: results of a prospective pilot and phase II study. Ann Oncol 2009;20:319-25. [Crossref] [PubMed]
- Thiel E, Korfel A, Martus P, et al. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): a phase 3, randomised, non-inferiority trial. Lancet Oncol 2010;11:1036-47. [Crossref] [PubMed]
- Pels H, Schmidt-Wolf IG, Glasmacher A, et al. Primary central nervous system lymphoma: results of a pilot and phase II study of systemic and intraventricular chemotherapy with deferred radiotherapy. J Clin Oncol 2003;21:4489-95. [Crossref] [PubMed]
- Chen C, Sun P, Cui J, et al. High-dose Methotrexate plus temozolomide with or without rituximab in patients with untreated primary central nervous system lymphoma: A retrospective study from China. Cancer Med 2019;8:1359-67. [Crossref] [PubMed]
- Ferreri AJ, Illerhaus G. The role of autologous stem cell transplantation in primary central nervous system lymphoma. Blood 2016;127:1642-9. [Crossref] [PubMed]
- Illerhaus G, Muller F, Feuerhake F, et al. High-dose chemotherapy and autologous stem-cell transplantation without consolidating radiotherapy as first-line treatment for primary lymphoma of the central nervous system. Haematologica 2008;93:147-8. [Crossref] [PubMed]
- Omuro A, Correa DD, DeAngelis LM, et al. R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood 2015;125:1403-10. [Crossref] [PubMed]
- Schorb E, Kasenda B, Atta J, et al. Prognosis of patients with primary central nervous system lymphoma after high-dose chemotherapy followed by autologous stem cell transplantation. Haematologica 2013;98:765-70. [Crossref] [PubMed]
- Soussain C, Choquet S, Fourme E, et al. Intensive chemotherapy with thiotepa, busulfan and cyclophosphamide and hematopoietic stem cell rescue in relapsed or refractory primary central nervous system lymphoma and intraocular lymphoma: a retrospective study of 79 cases. Haematologica 2012;97:1751-6. [Crossref] [PubMed]
- Schorb E, Kasenda B, Ihorst G, et al. High-dose chemotherapy and autologous stem cell transplant in elderly patients with primary CNS lymphoma: a pilot study. Blood Adv 2020;4:3378-81. [Crossref] [PubMed]
- Kondo E, Ikeda T, Izutsu K, et al. High-dose chemotherapy with autologous stem cell transplantation in primary central nervous system lymphoma: data from the japan society for hematopoietic cell transplantation registry. Biol Blood Marrow Transplant 2019;25:899-905. [Crossref] [PubMed]
- Lee TH, Lee JH, Chang JH, et al. Reduced-dose whole-brain radiotherapy with tumor bed boost after upfront high-dose methotrexate for primary central nervous system lymphoma. Radiat Oncol J 2020;38:35-43. [Crossref] [PubMed]
- Korfel A, Thiel E, Martus P, et al. Randomized phase III study of whole-brain radiotherapy for primary CNS lymphoma. Neurology 2015;84:1242-8. [Crossref] [PubMed]
- Correa DD, Rocco-Donovan M, DeAngelis LM, et al. Prospective cognitive follow-up in primary CNS lymphoma patients treated with chemotherapy and reduced-dose radiotherapy. J Neurooncol 2009;91:315-21. [Crossref] [PubMed]
- Prica A, Chan K, Cheung MC. Combined modality therapy versus chemotherapy alone as an induction regimen for primary central nervous system lymphoma: a decision analysis. Br J Haematol 2012;158:600-7. [Crossref] [PubMed]
- Omuro AM, Ben-Porat LS, Panageas KS, et al. Delayed neurotoxicity in primary central nervous system lymphoma. Arch Neurol 2005;62:1595-600. [Crossref] [PubMed]
- Correa DD, Maron L, Harder H, et al. Cognitive functions in primary central nervous system lymphoma: literature review and assessment guidelines. Ann Oncol 2007;18:1145-51. [Crossref] [PubMed]
- Doolittle ND, Korfel A, Lubow MA, et al. Long-term cognitive function, neuroimaging, and quality of life in primary CNS lymphoma. Neurology 2013;81:84-92. [Crossref] [PubMed]
- Adhikari N, Biswas A, Gogia A, et al. A prospective phase II trial of response adapted whole brain radiotherapy after high dose methotrexate based chemotherapy in patients with newly diagnosed primary central nervous system lymphoma-analysis of acute toxicity profile and early clinical outcome. J Neurooncol 2018;139:153-66. [Crossref] [PubMed]
- Schreck KC, Grossman SA. Role of temozolomide in the treatment of cancers involving the central nervous system. Oncology (Williston Park) 2018;32:555-60, 569. [PubMed]
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009;10:459-66. [Crossref] [PubMed]
- Baumert BG, Hegi ME, van den Bent MJ, et al. Temozolomide chemotherapy versus radiotherapy in high-risk low-grade glioma (EORTC 22033-26033): a randomised, open-label, phase 3 intergroup study. Lancet Oncol 2016;17:1521-32. [Crossref] [PubMed]
- Rudà R, Bosa C, Magistrello M, et al. Temozolomide as salvage treatment for recurrent intracranial ependymomas of the adult: a retrospective study. Neuro Oncol 2016;18:261-8. [Crossref] [PubMed]
- Fisher BJ, Hu C, Macdonald DR, et al. Phase 2 study of temozolomide-based chemoradiation therapy for high-risk low-grade gliomas: preliminary results of Radiation Therapy Oncology Group 0424. Int J Radiat Oncol Biol Phys 2015;91:497-504. [Crossref] [PubMed]
- Portnow J, Badie B, Chen M, et al. The neuropharmacokinetics of temozolomide in patients with resectable brain tumors: potential implications for the current approach to chemoradiation. Clin Cancer Res 2009;15:7092-8. [Crossref] [PubMed]
- Herrlinger U, Kuker W, Platten M, et al. First-line therapy with temozolomide induces regression of primary CNS lymphoma. Neurology 2002;58:1573-4. [Crossref] [PubMed]
- Reni M, Zaja F, Mason W, et al. Temozolomide as salvage treatment in primary brain lymphomas. Br J Cancer 2007;96:864-7. [Crossref] [PubMed]
- Makino K, Nakamura H, Hide T, et al. Salvage treatment with temozolomide in refractory or relapsed primary central nervous system lymphoma and assessment of the MGMT status. J Neurooncol 2012;106:155-60. [Crossref] [PubMed]
- Faivre G, Butler MJ, Le I, et al. Temozolomide as a single agent maintenance therapy in elderly patients with primary CNS lymphoma. Clin Lymphoma Myeloma Leuk 2019;19:665-9. [Crossref] [PubMed]
- Gupta T, Mohanty S, Moiyadi A, et al. Factors predicting temozolomide induced clinically significant acute hematologic toxicity in patients with high-grade gliomas: a clinical audit. Clin Neurol Neurosurg 2013;115:1814-9. [Crossref] [PubMed]
- Houillier C, Ghesquieres H, Chabrot C, et al. Rituximab, methotrexate, procarbazine, vincristine and intensified cytarabine consolidation for primary central nervous system lymphoma (PCNSL) in the elderly: a LOC network study. J Neurooncol 2017;133:315-20. [Crossref] [PubMed]
- Kurzwelly D, Glas M, Roth P, et al. Primary CNS lymphoma in the elderly: temozolomide therapy and MGMT status. J Neurooncol 2010;97:389-92. [Crossref] [PubMed]
- Pels H, Juergens A, Glasmacher A, et al. Early relapses in primary CNS lymphoma after response to polychemotherapy without intraventricular treatment: results of a phase II study. J Neurooncol 2009;91:299-305. [Crossref] [PubMed]
- Langner-Lemercier S, Houillier C, Soussain C, et al. Primary CNS lymphoma at first relapse/progression: characteristics, management, and outcome of 256 patients from the French LOC network. Neuro Oncol 2016;18:1297-303. [Crossref] [PubMed]
- Lionakis MS, Dunleavy K, Roschewski M, et al. Inhibition of B cell receptor signaling by ibrutinib in primary CNS lymphoma. Cancer Cell 2017;31:833-43.e5. [Crossref] [PubMed]
- Chamoun K, Choquet S, Boyle E, et al. Ibrutinib monotherapy in relapsed/refractory CNS lymphoma: a retrospective case series. Neurology 2017;88:101-2. [Crossref] [PubMed]
- Soussain C, Choquet S, Houillier C, et al. Ibrutinib in relapse or refractory primary CNS and vitreo-retinal lymphoma. results of the primary end-point of the ILOC phase II study from the LYSA and the French LOC network. Hematol Oncol 2017;35:72. [Crossref]
- Grommes C, Pastore A, Gavrilovic I, et al. Single-agent ibrutinib in recurrent/refractory central nervous system lymphoma. Blood 2016;128:783. [Crossref]
- Grommes C, Wolfe J, Gavrilovic I, et al. Phase II of single-agent ibrutinib in recurrent/refractory primary (PCNSL) and secondary CNS lymphoma (SCNSL). Blood 2018;132:2965. [Crossref]
- Ghesquières H, Houillier C, Chinot O, et al. Rituximab-Lenalidomide (REVRI) in Relapse or Refractory Primary Central Nervous System (PCNSL) or Vitreo Retinal Lymphoma (PVRL): results of a "Proof of Concept" Phase II Study of the French LOC Network. Blood 2016;128:785. [Crossref]
- Nelson DF, Martz KL, Bonner H, et al. Non-Hodgkin's lymphoma of the brain: can high dose, large volume radiation therapy improve survival? Report on a prospective trial by the Radiation Therapy Oncology Group (RTOG): RTOG 8315. Int J Radiat Oncol Biol Phys 1992;23:9-17. [Crossref] [PubMed]
- Laack NN, Ballman KV, Brown PB, et al. Whole-brain radiotherapy and high-dose methylprednisolone for elderly patients with primary central nervous system lymphoma: Results of North Central Cancer Treatment Group (NCCTG) 96-73-51. Int J Radiat Oncol Biol Phys 2006;65:1429-39. [Crossref] [PubMed]
- Schuurmans M, Bromberg JE, Doorduijn J, et al. Primary central nervous system lymphoma in the elderly: a multicentre retrospective analysis. Br J Haematol 2010;151:179-84. [Crossref] [PubMed]
- Song J, Samant R, Jay M, et al. Whole brain radiotherapy improves survival outcomes in primary CNS lymphoma patients ineligible for systemic therapy. Support Care Cancer 2020;28:5363-9. [Crossref] [PubMed]
- Kobayashi H, Yamaguchi S, Motegi H, et al. Long-term evaluation of combination treatment of single agent HD-MTX chemotherapy up to three cycles and moderate dose whole brain irradiation for primary CNS lymphoma. J Chemother 2019;31:35-41. [Crossref] [PubMed]
- Fossard G, Ferlay C, Nicolas-Virelizier E, et al. Utility of post-therapy brain surveillance imaging in the detection of primary central nervous system lymphoma relapse. Eur J Cancer 2017;72:12-9. [Crossref] [PubMed]
- Mylam KJ, Michaelsen TY, Hutchings M, et al. Little value of surveillance magnetic resonance imaging for primary CNS lymphomas in first remission: results from a Danish Multicentre Study. Br J Haematol 2017;176:671-3. [Crossref] [PubMed]
- Richard NM, Bernstein LJ, Mason WP, et al. Cognitive rehabilitation for executive dysfunction in brain tumor patients: a pilot randomized controlled trial. J Neurooncol 2019;142:565-75. [Crossref] [PubMed]
Cite this article as: Martinez-Calle N, Isbell LK, Cwynarski K, Schorb E. Treatment of elderly patients with primary CNS lymphoma. Ann Lymphoma 2021;5:2.


