Antibiotic treatment for bacteria-associated extranodal marginal zone lymphoma
Introduction
Marginal zone lymphomas (MZL) represent approximately 8% of all non-Hodgkin lymphomas (NHL) (1), spanning at least three different entities (2): splenic MZL, nodal MZL and extranodal MZL of mucosa-associated lymphoid tissue (MALT). The first two forms are rare, representing less than 2% of all NHL, while the extranodal MZL represents the most commonly occurring MZL subtype (3).
MZL is an interesting model of antigen-driven malignancy, where factors related to host and environment show a critical role in the pathogenesis of the disease. Extranodal MZL usually arises in the context of chronic inflammation related to infective agents as in Helicobacter pylori (Hp)-induced gastritis or following autoimmune disorders like Sjögren syndrome or Hashimoto thyroiditis (4). Since the discovery of pathogenic association between Hp infection and gastric MALT lymphoma (5,6), other infective agents have been investigated such as Chlamydia psittaci (Cp) in ocular adnexal MALT lymphomas (7) and Borrelia burgdorferi (Bb) in cutaneous MZL (8). Some other bacteria with putative lymphomagenesis capability have been proposed. Level of evidence supporting these associations is variable, with only a few of them achieving the Koch’s postulates, currently used to define causative agents of defined diseases. In the last 20 years, the role of Koch’s postulates to establish causative agents of neoplastic disease have been revised, and the concept of multifactoriality into the process of lymphomagenesis has been introduced (9,10). Melenotte et al. recently proposed a further change in the identification of causality relationships between bacteria and neoplasm (11), proposing a list of eight criteria to support the link: epidemiological, consistency among repeated observations, temporality, anatomical proximity, in situ localization of the bacteria within the NHL biopsy samples, experimental in vitro B-cell transformation, in vivo development of lymphoma, and lymphoma remission after antibiotic therapy. Only Hp infection complies with all the points illustrated, highlighting the difficulty of identifying universal criteria to point out a role of the infectious agent in the lymphomagenesis process.
Importantly, the prevalence of these associations can change in different periods and among diverse geographical areas, which has been largely reported also for viruses with lymphomagenic potentialities as Epstein-Barr virus (EBV) and hepatitis C virus (HCV) (12-15).
The pathogenic role of some bacteria resulted in the use of antibiotics to eradicate infections and to avoid the antigenic triggering that maintain lymphoma cells survival and growth. Following the example of Hp-associated gastric MALT lymphoma, antibiotic therapy gains space in the field of biological anti-lymphoma treatments, but level of evidence supporting associations and efficacy of antibiotic therapy is variable. Herein, we review the ethio-pathogenic associations between extranodal MZLs and causative bacteria and their therapeutic implications, discussing critically available evidence supporting lymphomagenesis and treatment of these combinations, with the aim to provide diagnostic and therapeutic recommendations and to analyze future perspectives in this important field of research.
The diagnosis of MZL relies on histopathological evaluation according to the criteria established by current WHO classification (2). Neoplastic lesions show a diffuse infiltrate of lymphocytes cells with a variable amount of centrocytic-like, small or monocytoid looking cells (16). Neoplastic lymphocytes are immunoreactive for CD20, CD79a, Bcl2, IgM, in a variable proportion of cases for IRTA1, MNDA, CD23, and CD43 and negative for cyclin D1, CD5, CD10, and IgD (4). Evaluation of CD5 and CycD1 are critical to exclude mantle cell lymphoma, even if a small proportion of CD5-positive EMZL has been reported (17,18). The occurrence of lymphoepithelial lesions, albeit characteristic, is not pathognomonic for MALT lymphomas, since they can also be present in reactive conditions or other lymphomas.
Helicobacter pylori
Hp is a spiral shaped gram-negative bacterium, classified in 1994 as a group 1 carcinogen by the International Agency for Research on Cancer (19). This bacterium colonizes the gastric mucosa with an incidence of 80% in developing countries and 50% in industrialized countries and is responsible of active gastritis that have been associated with the development of gastric adenocarcinoma or lymphoma. The route by which infection occurs remains unknown (20,21). Person-to-person transmission of Hp through either fecal/oral or oral/oral exposure seems most likely (21,22). Humans appear to be the major reservoir of infection; however, Hp has been also isolated from primates in captivity (23).
In physiological conditions, the gastric mucosa does not contain lymphoid tissues, but, in Hp-induced gastritis, features of acquired MALT are observed. The prevalence of Hp infection in 92% of patients with gastric MALT lymphoma (24,25), as well as tumor regression after Hp eradication with specific antibiotics in 75% of patients with gastric MALT lymphoma are important data supporting the relationship between this bacterium and gastric lymphomagenesis (26).
In addition, molecular studies performed in patients with Hp gastritis and subsequent gastric MALT lymphoma detected the same B-cell clone in both diagnostic samples, suggesting a multistep process initiated by Hp infection that can lead to lymphoma development eventually (27).
Monoclonality in patients with Hp-related gastritis has been reported as a precursor of MALT lymphoma development (28). However, many of those patients will never develop MALT-lymphoma. Thus, clonality assessment should not be performed in the absence of clear histological evidence of MALT-lymphoma, and should not be used for patients’ follow-up.
All these findings suggest that lymphoma development is a consequence not only of host dependent factors, but also of Hp-related factors, such as expression of cytotoxin-associated gene A (CagA) (29). The latter has been implicated not only in the general virulence of the strains, but also in their ability to induce lymphomas and solid tumors. As in the gastric cancer, CagA expressed in B cells undergoes tyrosine phosphorylation and binds to SHP2 (30). CagA-deregulated SHP2 then aberrantly activates Erk, which in turn induces phosphorylation of Bad at Ser112 that hampers Bad/Bcl-2 interactions, enabling Bax/Bcl-2 heterodimers to form in lieu of pro-apoptotic Bax homodimers (31). CagA also inhibits apoptosis of B cells by simultaneously affecting the function of p53 and the JAK/STAT signaling pathway (32).
These CagA activities may promote accumulation of preneoplastic B cells by subverting their elimination through apoptosis, which may additionally contribute to the development of MALT lymphoma (31).
The clinical presentation of gastric MZL is characterized by dyspepsia (90% of cases), epigastric pain, and anemia due to chronic bleeding, while B symptoms are rare. The endoscopic findings are not specific and vary from a flat gastritis appearance to one or more ulcers (33). Hp in histological specimens can be detected morphologically by hematoxylin-eosin or modified Giemsa stains, by immunostaining with specific monoclonal antibody, tissue culture, or PCR (34). Other noninvasive tests for Hp detection include urease breath test, assessment of CagA in the feces and determination of anti-Hp antibody titers in the serum.
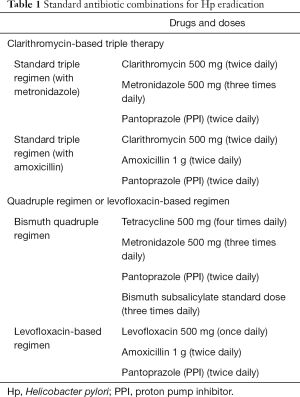
Hp eradication with wide-spectrum antibiotics is the conventional first-line treatment for patients with limited-stage gastric MALT lymphoma associated with this bacterial infection (35). The ideal eradication therapy should be safe, effective (eradication rate >90%), simple, and economical. With the wide application of antibiotics, drug resistance is increasing, and represents the main reason for treatment failure (36). The choice of antibiotics combination should depend on local drug resistance rates estimated in the respective country (Table 1). Primary and acquired resistance to clarithromycin and metronidazole has increased globally in the last years, leading to a loss of efficacy of the conventional first-line treatment regimens (36). Since clarithromycin represents the cornerstone of eradication therapy, resistance to this drug accounts for the main determinant of antimicrobial treatment: in countries where the incidence of resistance is low (i.e., <15%), current first-line standard regimen is a triple therapy with clarithromycin in association with metronidazole or amoxicillin and a proton pump inhibitor (PPI) or a bismuth quadruple therapy (37-39). The second line therapy should then be the bismuth-quadruple or a triple therapy containing fluoroquinolones (40). In countries where the incidence of macrolides resistance is higher, resistance to metronidazole should be considered as well, and, where it is low, a triple therapy with PPI, amoxicillin and metronidazole can be used. If the resistance for both clarithromycin and metronidazole is low, bismuth quadruple or a concomitant non-bismuth quadruple therapy should be used (37,40).
Full table
The increasing rate of resistance has led clinicians to modify therapies by choosing regimes including tetracycline with a PPI and a bismuth salt or the use of levofloxacin or rifabutin-based treatment regimens (37,41,42). However, combinations require high patient compliance, implying the intake of several tablets for 10–14 days. Incomplete adherence would further increase the onerous problem of resistance (43). In a hostile environment for its growth, Hp takes the coccoid form, a condition refractory to antibiotics. Coccoid forms can restore their activity 2 or more weeks after the end of treatment and they represent an important reason for both eradication failure and relapse (44), also considering their ability to survive for periods of time outside the cells in feces or in water.
Hp eradication induces lymphoma regression and long-term clinical disease control in 75% of patients (35). The length of time required to achieve a remission ranges from a few weeks to more than a year. In patients who achieve clinical and endoscopic remission with eradication of Hp but display persistent disease by histology, it is reasonable to wait for at least 12 months before starting another treatment (45,46). Upon documentation of Hp eradication, strict endoscopic follow-up is recommended, with multiple biopsies taken 2 and 3 months after treatment to prevent tumor progression, and subsequently (twice per year for 2 years) to monitor the histological regression of the lymphoma.
Two different mechanisms are responsible for the recurrence of Hp infection: recrudescence and reinfection. Recrudescence is the recurrence of the original bacterium after a failed attempt of eradication; reinfection occurs when, after a successful eradication, the patient shows an infection with either the original strain or a new strain of Hp (47). Many investigators have found that recurrence rates during the first 3–12 months after antibiotic therapy are due to late recrudescence whereas a Hp negativity for 1 year after treatment is a reliable indicator of successful eradication (48).
Definition of therapeutic response and choice of further treatment is based on the use of a histological grading system on post-treatment gastric biopsies (49) (Table 2). Accordingly, the detection of complete response (CR) or probable minimal residual disease (pMRD) after successful Hp eradication with conventional antibiotic therapy is considered as a state of remission that does not need for additional treatment. In the case of responding residual disease (rRD), a partial and ongoing response is thinkable and management should be personalized; endoscopic follow up can be adopted unless suspicious of tumor progression or presence of endoscopic unfavorable features. In the largest reported series, Hp eradication with triple antibiotic therapy has been followed by a CR and pMRD rates of 68% and 9%, respectively, with an overall response rate (ORR) of 77% in a multicenter cohort study of 420 patients with gastric MALT treated (50). One-quarter of the patients did not respond, with rRD in 3% and no change (NC) in 20%; the median time from Hp eradication to CR or pMRD was 4 months (range, 1–94). After a median follow-up period for responders (CR and pMRD) of 5.48 years (range, 1–14), only 3% of responders experienced lymphoma relapse.

Full table
Antibiotic therapy has been also proposed as first-line treatment to patients with localized Hp-negative gastric MALT lymphoma, which is recommended also by international guidelines. In a systematic review on 110 patients with Hp-negative gastric MALT lymphoma (51), front-line antibiotic therapy has been associated by a 15% complete remission rate, which has risen up to 38% in a few small, single-center series (52-54). These findings could be explained by false negative results in Hp diagnostic tests or by the involvement bacteria other than Hp in the development of gastric MALT lymphoma (46).
t(11,18)(p21;p21) is an important predictor of response to antibiotic therapy in Hp-related gastric MALT lymphoma. This translocation has been exclusively reported in MALT lymphomas and is common in gastric forms (24% of cases) (55). t(11,18)(p21;p21) is associated with lower responsivity to antibiotics, and has been detected in 47% of gastric localized MALT lymphoma unresponsive to Hp-eradicating therapy (46). Nevertheless, the assessment of this translocation is not strictly recommended in clinical practice (35).
Chlamydia psittaci
The Chlamydiae are obligate intracellular prokaryotic bacteria that infect mainly epithelial mucosa and are responsible for a great number of human infections involving not only mucosae but also the eye, genital tract, respiratory tract, and joints. These bacteria can inhibit apoptosis of infected cells and their oncogenic role is supported by the evidence of their ability to establish persistent infection, be mitogenic in vitro, induce polyclonal cell proliferation in vivo, and cause resistance to apoptosis in infected cells (56-58). Chlamydiae undergo an orderly alternation between a metabolically inactive, infective form [the elementary body (EB)] and a metabolically active intracellular growth stage form [the reticulate body (RB)]. This reversible growth option is referred to as persistence and is important in the pathogenesis of chronic chlamydial infections. Cp is the etiologic agent of psittacosis, a human infection caused by exposure to infected animals. Cp DNA has been detected in different percentages in several cases of EMZL arising in the ocular adnexal, skin, thyroid gland, salivary gland, and lung (59,60); however, to date, the highest incidence of Cp infection has been reported in MZL of ocular adnexal (OAMZL).
The pathogenic role of Cp in patients with OAMZL is supported by several evidence: the detection of its DNA in lymphoma samples and peripheral blood respectively in 80% and 40% of these patients (7), the recognition of both EB and RB of this bacteria in the macrophages of tumor samples by electronic microscopy, the isolation of Cp bodies in in vitro cultures from conjunctival swab and peripheral blood (61), and the regression of tumor lesion after Cp eradication with antibiotic therapy (62-65). As reported for other pathogens, chronic stimulation due to Cp infection may provide antigens able to induce immune reactions cross-reacting with host self-antigen (61,66), leading to the loss of local tolerance, and capability of eliminate inflammatory stimulus and promoting ultimately the onset of lymphoma (56).
The prevalence of Cp infection in OAMZL shows a striking variation among different geographical areas (58,67), probably also for methodological pitfalls, for the varied use of wide-spectrum antibiotics before biopsy, and the putative involvement of other microbial agents in lymphomagenesis (56). The highest association rates have been reported in some European countries and Korea, but large studies centrally assessing tissue samples of suitable series from several countries have not been performed.
Ocular adnexal lymphoma (OAL) represents 1–2% of all NHL and 5–15% of all extranodal NHL (68,69); with an incidence of approximately 0.28 per 100,000 subjects. OAMZL usually arises after the fourth decade, with a median age of 65 years and high prevalence among females (61). The clinical presentation of OAMZL depends on involved structures, with 25% showing conjunctival lesions, and intraorbital masses in 75% of cases. Conjunctival lesions appears as the typical “salmon red patch”, while intraorbital localization may cause exophthalmos (27% of cases), palpable masses (19%), ptosis (6%), diplopia (2%), orbital edema, epiphora (56).
The evidence of an association between Cp and development of OAMZL was demonstrated in a study published in 2004 (7). In this study, the presence of Cp DNA has been investigated by polymerase chain reaction in diagnostic biopsies from 40 patients with OAL, 20 non neoplastic orbital biopsies, 26 reactive lymphadenopathy samples, and peripheral blood mononuclear cells (PBMCs) from 21 lymphoma patients and 38 healthy individuals. Cp DNA has been detected in 80% of OALs, whereas has been absent in controls. Forty-three percent of patients with Chlamydia-positive lymphomas carried Cp DNA in their PBMCs, whereas none of the healthy donors carried Cp DNA in their PBMCs (43% vs. 0%; P<0.001).
Seven patients with chlamydia-positive OAMZL have been treated with the antibiotic doxycycline, and objective response has been assessed in four patients with measurable lymphoma lesions. One month after doxycycline treatment, chlamydial DNA has been no longer detectable in the PBMCs of all seven treated patients, and objective response has been observed in two of the four evaluable patients. In accordance with Koch’s first and second postulate, these data strengthen the association of pathogenicity between the presence of Cp and the onset of lymphoma, supporting further studies and a different therapeutic approach.
Several experiences of antibiotic therapy have been reported both at the diagnosis and at the relapse in patients with OAMZL (62,63,65,70). In a phase II trial, 27 patients with OAMZL at first or subsequent lines have been treated with a 3-week course of doxycycline (63). This strategy has been associated with an ORR of 48%, with responses in 64% of Cp DNA positive patients and 38% of Cp DNA negative patients. Responses have been recorded even among patients with regional lymphadenopathies and both in previously irradiated and non-irradiated patients. The 2-year failure-free survival was 66%. In 2012, the results of the first international phase II were published (64). Thirty-four patients with newly diagnosed OAMZL and measurable or parametrable disease have been treated with the 3-week course of doxycycline. Cp DNA has been detected in 89% of biopsies samples; 29 patients had Cp DNA in baseline swabs and/or blood samples and have been evaluable for chlamydial eradication, which has been achieved in 48% of patients at 1 year of follow-up. ORR has been 65%, with a 5-year PFS for the eradicated patients of 55%. Cp eradication has been associated with improved ORR and a better PFS. The latter findings have demonstrated that lymphoma regression is due to microorganism eradication and not to a direct anticancer property of the antibiotic (71). Patients with continuous contact with infected animal have experienced Cp reinfection compromising the effectiveness of antibiotic therapy (56).
In a retrospective series (70), Korean colleagues have analyzed data of 38 patients with OAMZL treated with front-line, single-course doxycycline at a dose of 100 mg twice a day for 3 weeks, or with a double course, with 3 weeks off, for patients with residual eye-related symptoms or non responders to a single course. An ORR of 47% (complete remission rate: 18%) has been recorded at a median follow up of 26.4 months, with a 3-year time-to-treatment failure rate of 84%. Moreover, patients treated with two courses of antibiotic showed a higher response rate compared to a single course (54% vs. 33%) and the same response rate has been recorded regardless Cp infection status. The same authors have conducted a study on 90 patients with newly-diagnosed OAMZL treated with two cycles of doxycycline (100 mg bid) for 3 weeks, reporting an ORR of 27% and a stability of the disease in 38% with a median PFS of 61 months (65). In contrast, no responses have been reported in a retrospective Austrian study on 11 patients with unknown Cp infection status, treated with doxycycline and followed for only 9 months (72). However, the small size of the cohort, the lack of Cp assessment and the very short follow up do not allow further consideration.
These data support an ongoing trial (IELSG39; ClinicalTrials.gov Identifier: NCT01820910) aimed to assess the efficacy of first-line Cp-eradicating therapy with protracted administration of doxycycline followed by eradication monitoring and antibiotic re-treatment at infection re-occurrence in this setting of patients. Patients are being treated with doxycycline 100 mg twice daily for four weeks followed by four weeks rest, repeated for three cycles. In the case of infection re-occurrence at any time (assessed 3 months after the end of therapy and then every six months by tests on conjunctival swabs and PBMC), patients are treated again with two courses of 4-week on, 4-week off of doxycycline 100 mg twice daily to prevent relapse. The results of this ongoing trial could provide us with further confirmation on the effectiveness of antibiotic therapy and treatment scheme as well as on the association of the infection with the recurrence of lymphoma.
Borrelia burgdorferi
Bb is a spirochetal bacterium that can be transmitted to man by Ixodes ticks and is the causative agent of Lyme disease. Acrodermatitis chronica atrophicans is a chronic cutaneous manifestation of Lyme disease and has been variably associated with primary cutaneous B-cell lymphomas (8,73,74); this association has been confirmed by serological tests and molecular studies from small retrospective series or case reports (8,73-77). Data about the prevalence of Bb in primary cutaneous lymphoma (PCL) are contrasting; this association ranges from 10% to 42% in some endemic areas (78) and has not been reported in other series from Europe, Asia or America (79-81).
A recent metanalysis shows an association between Bb and the onset of PCL, irrespectively of histological subtype; the limited figures of analyzed histotypes, however, prevents drawing any conclusion (82).
Bb infection might be associated with chronic antigen-driven lymphomagenesis in the skin, which is the portal of entry of this bacterium. Later on, lymphocytes may infiltrate the dermis and produce the characteristic Borrelial “lymphocytoma”. This lesion can be difficult to distinguish from MZL, which led experts to introduce the term “Borrelia-associated pseudolymphoma”, and requires monoclonality assessment to differentiate this entity from a true lymphoma (16).
Primary cutaneous MZL usually present as asymptomatic, solitary or multiple erythematous to brown-colored papules, nodules, or plaques; lesions are distributed over the trunk and extremities with predominance in the upper extremity; B symptoms are generally absent (83,84). Median age is from 39 to 55 years and overall, the prognosis is favorable, with 5-year overall survival cited at over 95% (85). Histological transformation into high-grade lymphoma is a rare event, spontaneously resolution has been described, but recurrence is frequent. The association between Bb infection and PCL should be assessed by anti-Bb IgG and IgM serology. In selected centers, with suitable expertise, ad hoc polymerase chain reaction techniques aimed to detect Bb on skin biopsy specimens are performed, but this procedure is not used in routine practice.
In patients with PCL diagnosed in endemic areas, antibiotic therapy aimed to eradicate Bb infection should be considered as the first-line option before proceeding to more aggressive treatments. Reported data on antibiotic therapy suggest a superiority of intravenous cephalosporin over the use of high doses of tetracycline per os (86). However, this conclusion is based on case reports or small retrospective case-series (76,87-92). Doxycycline 100 mg bid for at least 3 weeks (max 6 months) is the most widely used oral antibiotic, which is associated with varied results. Complete lymphoma remission has been more frequently reported among patients treated with intravenous cephalosporin (mostly ceftriaxone 2 g daily) for at least 2 weeks.
Achromobacter xylosoxidans (A. xylosoxidans )
A. xylosoxidans is a gram-negative beta-proteobacterium with low virulence but high resistance to antibiotic therapy, with imipenem and piperacillin being the most active drugs (93). Pulmonary parenchyma normally does not contain organized lymphoid tissue, but inflammatory stimuli such as follicular bronchiolitis, pulmonary inflammatory processes, smoking and acute infections may lead to bronchus-associated lymphoid tissue (BALT) acquisition (94,95). A single experience reported the association between A. xylosoxidans and BALT (96). The authors retrospectively analyzed 124 cases of pulmonary MALT lymphoma from different countries and a series of 81 control cases without evidence of lymphoma (normal lung, pneumonia, alveolitis, lung metastases, emphysema). They detected A. xylosoxidans in 46% of pulmonary MALT lymphomas with difference in the prevalence rate for the different geographical regions, ranging from 33% to 67%, with the presence of this bacterium being detected in only 18% of controls (P=0.004). As described for other pathogens, also A. xylosoxidans can play a role as trigger for chronic inflammation, but a definite causative role of this bacterium for the pathogenesis of pulmonary MALT lymphoma, however, cannot be assumed. A retrospective study on a Japanese series of 52 cases of pulmonary MZL has shown the presence of A. xylosoxidans DNA only in one case (2%) (97).
Activity of antibiotic therapy has been reported in only two patients with BALT-type lymphoma and in both cases the presence of A. xylosoxidans was not assessed (98). The first patient has been treated with chemotherapy (CHOP) and subsequent therapy with clarithromycin 200 mg daily obtaining a significant response to antibiotic therapy; the second patient had a concomitant Hp-positive gastritis and experienced MALT lymphoma regression immediately after the eradication of this bacteria, achieving a complete remission while receiving long-term clarithromycin at the same dosage. Microbiological investigations have been carried out in neither patient and the effect of clarithromycin could be secondary not only to antibacterial properties, but also to known antineoplastic capabilities (see below). Current microbiological knowledge about A. xylosoxidans is borrowed from the experience provided by patients with cystic fibrosis in which the bacterium is often isolated. This pathogen is not a highly virulent agent, but the well-documented multidrug resistance in cystic fibrosis patients seems to suggest that a poly-antimicrobial therapy may be needed to successfully eradicate this bacterium.
Campylobacter jejuni (Cj)
Cj is a gram-negative bacterium responsible of acute gastroenteritis worldwide, mostly in developing countries. Cj was associated with immune-proliferative small intestine disease (IPSID), an intestinal variant of the MALT lymphoma (16). The duodenum is the most common site of disease (63% of patients), followed by the jejunum (17%) and ileum (8%) (99). IPSID represents a single entity which may show a spectrum of different clinical aspects, including benign, intermediate and overtly malignant stages that have been formerly named “α-heavy chain disease” and “Mediterranean lymphoma”. Patients with small intestinal lymphoma of Northern Europe and America show relevant socio-epidemiologic, clinical, anatomical, and histopathological differences with respect to patients from the Mediterranean area. It is difficult to find cases of IPSID in its true “Mediterranean form” in Western people.
IPSID affects mainly older children and young adults (range, 10–35 years; median, 25–30 years) of low socioeconomic status in developing countries. It is uncommon in other age groups. Most cases of IPSID are reported in the Middle East, North and South Africa, and the Far East (100-102). Accordingly, most patients present with a malabsorption syndrome with colicky abdominal pain, diarrhea, severe weight loss, hypoproteinemia, and immunological deficits. In the advanced stage of this disease, vomiting, abdominal mass and organomegaly are more frequent (100). Endoscopy of the small intestine shows variable abnormalities including thickened mucosal folds, nodules, ulcers, and/or sub-mucosal infiltration whereby the intestine is motionless, firm to touch, and non-distensible. Barium X-rays of the small intestine show diffuse dilation of the organ, with thickened mucosal folds and spiculated fold edges.
Bacterial overgrowth and intestinal parasitosis (mainly with Giardia) are common, as are anemia and vitamins deficiencies. The number of circulating lymphocytes is reduced and humoral and cell-mediated immune responses are often impaired. The most relevant laboratory abnormality in IPSID is the detection, mainly during the early stages of the disease, in serum, urine, saliva, or intestinal secretions, of α-heavy chain, whereas Bence-Jones protein is characteristically absent. The α-heavy chain is a 29,000–34,000 kDa protein produced by intestinal plasma cells that carries an internal deletion of the VH and CH1 regions and is devoid of associated light chain. The distinguishing histopathological feature of IPSID is the presence of dense mucosal infiltrate mostly represented by many plasma cells and, to a lesser extent, by “centrocyte-like” predominantly involving long segments of the proximal portions of small bowel mucosa (102,103).
The presence of Cj has been demonstrated in the intestinal tissue obtained from a patient with IPSID who had a dramatic response to antibiotic and a retrospective analysis of archival intestinal biopsy specimen identified Campylobacter species in four of six additional patients with IPSID, using fluorescent in situ hybridization (FISH) and immunohistochemical techniques (104). Therefore, the role of Cj as an infectious trigger has been hypothesized and a current hypothesis is that in the context of impaired immunity, patients may have difficulties clearing Cj small bowel infection, resulting in chronic antigenic stimulation leading to a gut mucosa-associated, IgA-producing plasma cell proliferation leading to the development of IPSID (100,105).
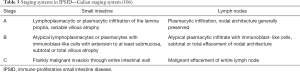
There is no consensus on the best therapeutic approach to IPSID patients. The experiences reported in literature are the result of limited and often retrospective case series. Stage of disease is one the most important factors determining the therapeutic choice. Two staging system exist: one is based on histologic findings (106) (Table 3) and the other based on the anatomic spread of the lymphomatous disease (107) (Table 4). In the early stages, authors agree on the use of broad-spectrum antibiotic therapy as exclusive treatment; outcome differ both in the choice and duration of the antibiotic therapy. The only available prospective study shows the results of 21 Tunisian patients treated between 1981 and 1985 (108). Six of these patients had early disease and have been treated with upfront antibiotic therapy. In the case of jejunal bacterial overgrowth, the patients have received antibiogram-selected antibiotics, while patients without bacterial overgrowth, have been treated with ampicillin plus metronidazole; duration of antibiotic therapy varied from 7 to 24 months. Patients with advanced stage have received anthracycline-based combination chemotherapy. The overall survival was 90%±12% at 2 years and 67%±25% at 3 years. All patients alive beyond 3.5 years were disease free.
Another experience from Turkish colleagues reported results from 23 patients (109) (16 stage B and C). Seven patients had stage-A IPSID and have been treated with upfront tetracycline monotherapy for a median duration of 7 months (range, 6–11); five of them had a complete remission and two had a partial response. The other 16 patients had a stage B or C IPSID and have been treated with COPP chemotherapy followed by tetracycline 1 g/d for 6 months in responding patients, obtaining a complete remission in 11 patients, with a 5-year disease-free survival of 75% and a 5-year OS of 70%.
Antibiotic therapy seems to be an effective and well tolerate therapeutic option as first-line treatment of patients with stage-A IPSID (110). The best regimen should include tetracycline with or without metronidazole for at least 6 months, with periodic response assessment to indicate maintenance treatment in patients who do not achieve a complete remission (102). For stage B and C, CHOP or CHOP-like chemotherapy should be used; an adequate supportive therapy should be initiated as well in case of clinical or laboratory signs of malabsorption and dehydration (110,111).
Antibiotics as anti-proliferating agents
In the last years, the role of antibiotics as antineoplastic agents inducing cell cycle arrest, apoptosis, autophagy, and oxidative stress was also investigated (112-115), and, in particular, macrolides have been tested in hematological malignancies. Several in vitro models of immunomodulatory and direct antiproliferative activity of macrolides have been reported, including increased activity of NK-cells, accumulation of cytotoxic CD8+ and interferon-gamma producing T-cells, inhibition of TNF- and VEGF activity and decrease of IL-6 and IL-8. Macrolides have also been shown to directly inhibit the mTOR pathway (116).
Several clinical experiences have shown encouraging response rates with the use of clarithromycin, alone or in combination with immunomodulatory compounds, in patients with multiple myeloma or Waldenström’s macroglobulinemia (117,118). A phase II trial has demonstrated that the combination of clarithromycin, lenalidomide and dexamethasone (BiRD regimen) is an effective first-line therapy, with manageable toxicity, for patients with multiple myeloma (117). This combination has been associated with an objective response rate of 90%, a combined stringent and conventional CR rate of 39%, and 74% of the patients achieving at least a 90% decrease in M-protein levels. Thromboembolic events, corticosteroid-related morbidity and cytopenias have been the most relevant adverse events. The combination termed BLT-D (clarithromycin 500 mg orally twice daily, low-dose thalidomide 50 mg orally escalated to 200 mg daily, and dexamethasone 40 mg orally once weekly) has been associated with 83% response rate and a median time on therapy of 7 months (range, 3 to 28) in 12 patients with relapsed/refractory Waldenström’s macroglobulinemia (118). Neurotoxicity has been the major limiting adverse event, probably attributable to both thalidomide and paraprotein-related neuropathy; other toxicities included gastrointestinal and endocrinal manifestations, mostly of grade 1–2. BLT-D appeared to be an active, non myelosuppressive treatment for Waldenström’s macroglobulinemia, however, burdened by neurotoxicity that limited the escalation dose of thalidomide.
On these notions, anticancer activities of clarithromycin have been subsequently investigated in patients with lymphomas, in particular with MZL (119-122). In a pivotal phase-II trial (119), 13 patients with refractory EMZL have been treated with clarithromycin 500 mg orally, twice daily, for 6 months. As expected, clarithromycin has been well tolerated, with no cases of adverse event grade >1, and has shown an ORR of 38% and a 2-year PFS of 58%±13%. In the same setting of patients, another phase II study (HDK trial) has been conducted to evaluate the efficacy of high-dose clarithromycin for a shorter course (120). Four courses of oral clarithromycin 2 g/day, days 1–14, every 21 days have been associated with an ORR of 52% and a 2-year PFS of 56%±10% in 23 patients with relapsed/refractory EMZL. Toxicity has been mild, with only two cases of nausea grade >2.
A retrospective analysis on 55 patients (47 in second or further line of therapy) with MALT lymphoma treated with clarithromycin have been performed to better define the most effective and best tolerated dosage and administration schedule (121). ORR has been 57% in 32 patients treated with a dose of 1 g/day, and 41% in the 23 patients treated with a dose of 2 g/day (P=0.28). The 3-year PFS and OS for the 47 patients with relapsed/refractory disease have been respectively 51% and 96% (95% CI: 91–100%). Tolerability was excellent, and there were no cases of high-grade transformation nor lymphoma-related deaths. With the limitations of a retrospective analysis, a long-lasting treatment with a daily dose of 1 g has been suggested for treatment of patients with relapsed MZL. A recent phase II trial failed to meet the primary endpoint in 16 patients with relapsed MALT lymphoma treated with azithromycin as a single agent (123). Oral azithromycin 1,500 mg once-weekly 4 times a month has been associated with excellent safety profile but, although pharmacological and in vitro evidence of activity (116,122,124,125), the trial has been interrupted because the achieved 25% of ORR was below the threshold of interest.
A recent retrospective analysis of real-life experience has confirmed activity and safety of clarithromycin monotherapy in 23 patients with MALT lymphoma, both at diagnosis and relapse, with an ORR of 48% and 2-year PFS of 53% (126).
All together these studies support the role of macrolides as anticancer agents in patients with EMZL, even in cases where a bacterial target is not identified.
Conclusions
An etio-pathogenic association with a few bacteria has been reported for some MZL entities. The indolent clinical behavior of these neoplasms, support the safe use of antibiotic therapy as a first-line therapy, in particular in cases of localized disease. Anti-Hp antibiotic therapy plays an undiscussed role as upfront treatment of patients with gastric MALT lymphoma. The rarity of extra-gastric MZL entities hampers to execute randomized trials, however, single-arm phase II trials suggest that antibiotics are safe and effective treatments for patients with OAMZL or IPSID. Some antibiotics can also be considered for their anti-cancer capabilities, demonstrated both in vitro and in vivo, even when a bacterial target is not identified. Further lab and clinical investigations are warranted in this intriguing field. Among many others, identification of new micro-organisms with lymphomagenic capability, assessment of new antibiotics and combinations of antibiotics and other anticancer agents will constitute important steps forward in the treatment of MZL.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Francesco Bertoni, Thomas Habermann, Davide Rossi, and Emanuele Zucca) for the series “Marginal Zone Lymphomas” published in Annals of Lymphoma. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aol-21-3). The series “Marginal Zone Lymphomas” was commissioned by the editorial office without any funding or sponsorship. AJMF serves as an unpaid editorial board member of Annals of Lymphoma from Mar 2020 to Feb 2022. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Thieblemont C, Berger F, Dumontet C, et al. Mucosa-associated lymphoid tissue lymphoma is a disseminated disease in one third of 158 patients analyzed. Blood 2000;95:802-6. [Crossref] [PubMed]
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127:2375-90. [Crossref] [PubMed]
- Zucca E, Bertoni F, Roggero E, et al. The gastric marginal zone B-cell lymphoma of MALT type. Blood 2000;96:410-9. [Crossref] [PubMed]
- Thieblemont C, Bertoni F, Copie-Bergman C, et al. Chronic inflammation and extra-nodal marginal-zone lymphomas of MALT-type. Semin Cancer Biol 2014;24:33-42. [Crossref] [PubMed]
- Eidt S, Stolte M, Fischer R. Helicobacter pylori gastritis and primary gastric non-Hodgkin’s lymphomas. J Clin Pathol 1994;47:436-9. [Crossref] [PubMed]
- Wotherspoon AC. Gastric lymphoma of mucosa-associated lymphoid tissue and Helicobacter pylori. Annu Rev Med 1998;49:289-99. [Crossref] [PubMed]
- Ferreri AJ, Guidoboni M, Ponzoni M, et al. Evidence for an association between Chlamydia psittaci and ocular adnexal lymphomas. J Natl Cancer Inst 2004;96:586-94. [Crossref] [PubMed]
- Goodlad JR, Davidson MM, Hollowood K, et al. Primary cutaneous B-cell lymphoma and Borrelia burgdorferi infection in patients from the Highlands of Scotland. Am J Surg Pathol 2000;24:1279-85. [Crossref] [PubMed]
- Evans AS. Causation and disease: a chronological journey. New York: Plenum Medical, 1993.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological agents. Volume 100 B. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum 2012;100:1-441. [PubMed]
- Melenotte C, Mezouar S, Mège JL, et al. Bacterial infection and non-Hodgkin lymphoma. Crit Rev Microbiol 2020;46:270-87. [Crossref] [PubMed]
- Kanda T, Yajima M, Ikuta K, et al. Epstein-Barr virus strain variation and cancer. Cancer Sci 2019;110:1132-9. [Crossref] [PubMed]
- Al-Salam S, John A, Daoud S, et al. Expression of Epstein-Barr virus in Hodgkin lymphoma in a population of United Arab Emirates nationals. Leuk Lymphoma 2008;49:1769-77. [Crossref] [PubMed]
- Gisbert JP, García-Buey L, Arranz R, et al. The prevalence of hepatitis C virus infection in patients with non-Hodgkin's lymphoma. Eur J Gastroenterol Hepatol 2004;16:135-8. [Crossref] [PubMed]
- Torres HA, Nevah MI, Barnett BJ, et al. Hepatitis C virus genotype distribution varies by underlying disease status among patients in the same geographic region: a retrospective multicenter study. J Clin Virol 2012;54:218-22. [Crossref] [PubMed]
- Ponzoni M, Ferreri AJ. Bacteria associated with marginal zone lymphomas. Best Pract Res Clin Haematol 2017;30:32-40. [Crossref] [PubMed]
- Jaso JM, Yin CC, Wang SA, et al. Clinicopathologic features of CD5-positive nodal marginal zone lymphoma. Am J Clin Pathol 2013;140:693-700. [Crossref] [PubMed]
- Dreyling M, Thieblemont C, Gallamini A, et al. ESMO Consensus conferences: guidelines on malignant lymphoma. part 2: marginal zone lymphoma, mantle cell lymphoma, peripheral T-cell lymphoma. Ann Oncol 2013;24:857-77. [Crossref] [PubMed]
- Villain P, Gonzalez P, Almonte M, et al. European code against cancer 4th edition: infections and cancer. Cancer Epidemiol 2015;39:S120-38.
- Cave DR. Transmission and epidemiology of Helicobacter pylori. Am J med 1996;100:12S-7S. [Crossref] [PubMed]
- Mégraud F. Transmission of Helicobacter pylori: faecal-oral versus oral-oral route. Aliment Pharmacol Ther 1995;9:85-91. [PubMed]
- Perry S, Sanchez M, Yang S, et al. Gastroenteritis and transmission of Helicobacter pylori infection in households Emerg Infect Dis 2006;12:1701-8. [Crossref] [PubMed]
- Semrau A, Gerold S, Frick JS, et al. Non-invasive detection and successful treatment of a Helicobacter pylori infection in a captive rhesus macaque. Lab Anim 2017;51:208-11. [Crossref] [PubMed]
- Doglioni C, Wotherspoon AC, Moschini A, et al. High incidence of primary gastric lymphoma in northeastern Italy. Lancet 1992;339:834-5. [Crossref] [PubMed]
- Parsonnet J. Gastric adenocarcinoma and Helicobacter pylori infection. West J Med 1994;161:60. [PubMed]
- Wotherspoon AC, Doglioni C, Diss TC, et al. Regression of low grade B-cell gastric lymphoma of MALT type following eradication of Helicobacter pylori. Lancet 1993;342:575-7. [Crossref] [PubMed]
- Zucca E, Roggero E, Pileri S. B-cell lymphoma of MALT type: a review with spe-cial emphasis on diagnostic and management problems of low-grade gastrictumours. Br J Haematol 1998;100:3-14. [Crossref] [PubMed]
- Sorrentino D, Ferraccioli GF, S, DeVita S, et al. B-cell clonality and infection with Helicobacter pylori: implications for development of gastric lymphoma. Gut 1996;38:837-40. [Crossref] [PubMed]
- Schumausser B, Eck M, Greiner A, et al. Mucosal humoral immune reponse to CagA shows a high prevalence in patients with gastric MALT-type lymphoma. Virchow Arch 2000;436:115-18. [Crossref]
- Kuo SH, Chen LT, Lin CW, et al. Detection of the Helicobacter pylori CagA protein in gastric mucosa-associated lymphoid tissue lymphoma cells: clinical and biological significance. Blood Cancer J 2013;3:e125 [Crossref] [PubMed]
- Zhu Y, Wang C, Huang J, et al. The Helicobacter pylori virulence factor CagA promotes Erk1/2-mediated Bad phosphorylation in lymphocytes: a mechanism of CagA-inhibited lymphocyte apoptosis. Cellular Microbiology 2007;9:952-61. [Crossref] [PubMed]
- Umehara S, Higashi H, Ohnishi N, et al. Effects of Helicobacter pylori CagA protein on the growth and survival of B lymphocytes, the origin of MALT lymphoma. Oncogene 2003;22:8337-42. [Crossref] [PubMed]
- Levy M, Copie-Bergman C, Traulle C, et al. Conservative treatment of primary gastric low-grade B-cell lymphoma of mucosa-associated lymphoid tissue: predictive factors of response and outcome. Am J Gastroenterol 2002;97:292-7. [Crossref] [PubMed]
- Oleastro M, Menard A, Santos A, et al. Real-time PCR assay for rapid and accurate detection of point mutations conferring resistance to clarithromycin in Helicobacter pylori. J Clin Microbiol 2003;41:397-402. [Crossref] [PubMed]
- Zucca E, Arcaini L, Buske C, et al. Marginal zone lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2020;31:17-29. [Crossref] [PubMed]
- Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut 2010;59:1143-53. [Crossref] [PubMed]
- Malfertheiner P, Megraud F, O'Morain CA, et al. European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017;66:6-30. [Crossref] [PubMed]
- Gisbert JP, González L, Calvet X, et al. Proton pump inhibitor, clarithromycin and either amoxycillin or nitroimidazole: a meta-analysis of eradication of Helicobacter pylori. Aliment Pharmacol Ther 2000;14:1319-28. [Crossref] [PubMed]
- Fallone CA, Moss SF, Malfertheiner P. Reconciliation of recent helicobacter pylori treatment guidelines in a time of increasing resistance to antibiotics. Gastroenterology 2019;157:44-53. [Crossref] [PubMed]
- Pohl D, Keller PM, Bordier V, et al. Review of current diagnostic methods and advances in Helicobacter pylori diagnostics in the era of next generation sequencing. World J Gastroenterol 2019;25:4629-60. [Crossref] [PubMed]
- Toracchio S, Capodicasa S, Soraja DB, et al. Rifabutin based triple therapy for eradication of H. pylori primary and secondary resistant to tinidazole and clarithromycin. Dig Liver Dis 2005;37:33-8. [Crossref] [PubMed]
- Gisbert JP, Bermejo F, Castro-Fernández M, et al. H. pylori Study Group of the Asociación Española de Gastroenterología. Second-line rescue therapy with levofloxacin after H. pylori treatment failure: a Spanish multicenter study of 300 patients. Am J Gastroenterol 2008;103:71-6. [Crossref] [PubMed]
- Ford AC, Malfertheiner P, Giguere M, et al. Adverse events with bismuth salts for Helicobacter pylori eradication: systematic review and meta-analysis. World J Gastroenterol 2008;14:7361-70. [Crossref] [PubMed]
- Bode G, Mauch F, Malfertheiner P. The coccoid forms of Helicobacter pylori. Criteria for their viability. Epidemiol Infect 1993;111:483-90. [Crossref] [PubMed]
- Zucca E, Bertoni F. The spectrum of MALT lymphoma at different sites: biological and therapeutic relevance. Blood 2016;127:2082-92. [Crossref] [PubMed]
- Ruskoné-Fourmestraux A, Fischbach W, Aleman BM, et al. EGILS consensus report. Gastric extranodal marginal zone B-cell lymphoma of MALT. Gut 2011;60:747-58. [Crossref] [PubMed]
- Sachs G, Scott DR H.. Pylori: eradication or preservation? F1000 Medicine Reports 2012;4:7. [PubMed]
- Kayali S, Manfredi M, Gaiani F, et al. Helicobacter pylori, transmission routes and recurrence of infection: state of the art. Acta Biomed 2018;89:72-6. [PubMed]
- Copie-Bergman C, Wotherspoon AC, Capella C, et al. Gela histological scoring system for post-treatment biopsies of patients with gastric MALT lymphoma is feasible and reliable in routine practice. Br J Haematol 2013;160:47-52. [Crossref] [PubMed]
- Nakamura S, Sugiyama T, Matsumoto T, et al. Long-term clinical outcome of gastric MALT lymphoma after eradication of Helicobacter pylori: a multicentre cohort follow-up study of 420 patients in Japan. Gut 2012;61:507-13. [Crossref] [PubMed]
- Zullo A, Hassan C, Ridola L, et al. Eradication therapy in Helicobacter pylori-negative, gastric low-grade mucosa-associated lymphoid tissue lymphoma patients: a systematic review. J Clin Gastroenterol 2013;47:824-7. [Crossref] [PubMed]
- Kuo SH, Yeh KH, Wu MS, et al. First-line antibiotic therapy in Helicobacter pylori-negative low-grade gastric mucosa-associated lymphoid tissue lymphoma. Sci Rep 2017;7:14333. [Crossref] [PubMed]
- Kuo SH, Wu MS, Yeh KH, et al. Novel insights of lymphomagenesis of helicobacter pylori-dependent gastric mucosa-associated lymphoid tissue lymphoma. Cancers (Basel) 2019;11:547. [Crossref] [PubMed]
- Raderer M, Wöhrer S, Kiesewetter B, et al. Antibiotic treatment as sole management of Helicobacter pylori-negative gastric MALT lymphoma: a single center experience with prolonged follow-up. Ann Hematol 2015;94:969-73. [Crossref] [PubMed]
- Liu H, Ye H, Ruskone-Fourmestraux A, et al. T(11;18) is a marker for all stage gastric MALT lymphomas that will not respond to H. pylori eradication. Gastroenterology 2002;122:1286-94. [Crossref] [PubMed]
- Ferreri AJ, Dolcetti R, Du MQ, et al. Ocular adnexal MALT lymphoma: an intriguing model for antigen-driven lymphomagenesis and microbial-targeted therapy. Ann Oncol 2008;19:835-46. [Crossref] [PubMed]
- Chanudet E, Zhou Y, Bacon CM, et al. Chlamydia psittaci is variably associated with OAML in different geographical regions. J Pathol 2006;209:344-51. [Crossref] [PubMed]
- Ferreri AJ, Dolcetti R, Magnino S, et al. Chlamydial infection: the link with ocular adnexal lymphoma. Nat Rev Clin Oncol 2009;6:658-69. [Crossref] [PubMed]
- Chanudet E, Adam P, Nicholson AG, et al. Chlamydiae and Mycoplasma infections in pulmonary MALT lymphoma. Br J Cancer 2007;97:949-51. [Crossref] [PubMed]
- Aigelsreiter A, Gerlza T, Deutsch AJ, et al. Chlamydia psittaci infection in non gastrointestinal extranodal MALT lymphomas and their precursor lesions. Am J Clin Pathol 2011;135:70-5. [Crossref] [PubMed]
- Ferreri AJ, Dolcetti R, Dognini GP, et al. Chlamydophila psittaci is viable and infectious in the conjunctiva and peripheral blood of patients with ocular adnexal lymphoma: results of a single center prospective case-control study. Int J Cancer 2008;123:1089-93. [Crossref] [PubMed]
- Ferreri AJ, Ponzoni M, Dolcetti R, et al. Regression of ocular adnexal lymphoma after chlamydia psittaci-eradicating antibiotic therapy. J Clin Oncol 2005;23:5067-73. [Crossref] [PubMed]
- Ferreri AJ, Ponzoni M, Dolcetti R, et al. Bacteria-eradicating therapy with doxycycline in ocular adnexal MALT lymphoma: a multicenter prospective trial. J Natl Cancer Inst 2006;98:1375-82. [Crossref] [PubMed]
- Ferreri AJ, Govi S, Dolcetti R, et al. Chlamydophila psittaci eradication with doxycycline as first-line targeted therapy for ocular adnexae lymphoma: final results of an international phase II trial. J Clin Oncol 2012;30:2988-94. [Crossref] [PubMed]
- Han JJ, Kim TM, Heo DS, et al. Long-term outcomes of first-line treatment with doxycycline in patients with previously untreated ocular adnexal marginal zone B cell lymphoma. Ann Hematol 2015;94:575-81. [Crossref] [PubMed]
- Oldstone MB. Molecular mimicry and immune-mediated diseases. FASEB J 1998;12:1255-65. [Crossref] [PubMed]
- Bertoni F, Zucca E. State-of-the-art therapeutics: marginal-zone lymphoma. J Clin Oncol 2005;23:6415-20. [Crossref] [PubMed]
- Freeman C, Berg JW, Cutler SJ. Occurrence and prognosis of extranodal lymphomas. Cancer 1972;29:252-60. [Crossref] [PubMed]
- Fung CY, Tarbell NJ, Lucarelli MJ, et al. Ocular adnexal lymphoma: clinical behavior of distinct World Health Organization classification subtypes. Int J Radiat Oncol Biol Phys 2003;57:1382-91. [Crossref] [PubMed]
- Kim TM, Kim KH, Lee MJ, et al. First-line therapy with doxycycline in ocular adnexal mucosa-associated lymphoid tissue lymphoma: a retrospective analysis of clinical predictors. Cancer Sci 2010;101:1199-203. [Crossref] [PubMed]
- Ferreri AJ, Govi S, Ponzoni M. Marginal zone lymphoma and infectious agents. Semin Cancer Biol 2013;23:431-40. [Crossref] [PubMed]
- Grünberger B, Hauff W, Lukas J, et al. ‘Blind’ antibiotic treatment targeting Chlamydia is not effective in patients with MALT lymphoma of the ocular adnexa. Ann Oncol 2006;17:484-7. [Crossref] [PubMed]
- Cerroni L, Zöchling N, Pütz B, et al. Infection by Borrelia burgdorferi and cutaneous B-cell lymphoma. J Cutan Pathol 1997;24:457-61. [Crossref] [PubMed]
- Garbe C, Stein H, Dienemann D, et al. Borrelia burgdorferi-associated cutaneous B cell lymphoma: clinical and immunohistologic characterization of four cases. J Am Acad Dermatol 1991;24:584-90. [Crossref] [PubMed]
- Goos M, Roelcke D, Schnyder UW. Acrodermatitis chronica atrophicans with fibrous nodules and monoclonal gammopathy. Arch Dermatol Forsch 1971;241:122-33. [Crossref] [PubMed]
- Kütting B, Bonsmann G, Metze D, et al. Borrelia burgdorferi-associated primary cutaneous B cell lymphoma: complete clearing of skin lesions after antibiotic pulse therapy or intralesional injection of interferon alfa-2a. J Am Acad Dermatol 1997;36:311-4. [Crossref] [PubMed]
- de la Fouchardiere A, Vandenesch F, Berger F. Borrelia-associated primary cutaneous MALT lymphoma in a non endemic region. Am J Surg Pathol 2003;27:702-3. [Crossref] [PubMed]
- Ferreri AJ, Ernberg I, Copie-Bergman C. Infectious agents and lymphoma development: molecular and clinical aspects. J Intern Med 2009;265:421-38. [Crossref] [PubMed]
- Wood GS, Kamath NV, Guitart J, et al. Absence of Borrelia burgdorferi DNA in cutaneous B-cell lymphomas from the United States. J Cutan Pathol 2001;28:502-7. [Crossref] [PubMed]
- Goteri G, Ranaldi R, Simonetti O, et al. Clinicopathological features of primary cutaneous B cell lymphomas from an academic regional hospital in central Italy: No evidence of Borrelia burgdorferi association. Leuk Lymphoma 2007;48:2184-8. [Crossref] [PubMed]
- Li C, Inagaki H, Kuo TT, et al. Primary cutaneous marginal zone B-cell lymphoma: A molecular and clinicopathologic study of 24 Asian cases. Am J Surg Pathol 2003;27:1061-9. [Crossref] [PubMed]
- Travaglino A, Varricchio S, Pace M, et al. Borrelia burgdorferi in primary cutaneous lymphomas: a systematic review and meta-analysis. J Dtsch Dermatol Ges 2020;18:1379-84. [Crossref] [PubMed]
- Hope CB, Pincus LB. Primary cutaneous B-cell lymphomas. Clin Lab Med 2017;37:547-74. [Crossref] [PubMed]
- Swerdlow SH. Cutaneous marginal zone lymphomas. Semin Diagn Pathol 2017;34:76-84. [Crossref] [PubMed]
- Malachowski SJ, Sun J, Chen PL, et al. Diagnosis and management of cutaneous B-cell lymphomas. Dermatol Clin 2019;37:443-54. [Crossref] [PubMed]
- Senff NJ, Noordijk EM, Kim YH, et al. European Organization for Research and Treatment of Cancer and International Society for Cutaneous Lymphoma consensus recommendations for the management of cutaneous B-cell lymphomas. Blood 2008;112:1600-9. [Crossref] [PubMed]
- Bogle MA, Riddle CC, Triana EM, et al. Primary cutaneous B-cell lymphoma. J Am Acad Dermatol 2005;53:479-84. PubMed. [Crossref] [PubMed]
- Roggero E, Zucca E, Mainetti C, et al. Eradication of Borrelia burgdorferi infection in primary marginal zone B-cell lymphoma of the skin. Hum Pathol 2000;31:263-8. [Crossref] [PubMed]
- Monari P, Farisoglio C, Calzavara Pinton PG. Borrelia burgdorferi-associated primary cutaneous marginal-zone B-cell lymphoma: a case report. Dermatology 2007;215:229-32. [Crossref] [PubMed]
- Golling P, Cozzio A, Dummer R, et al. Primary cutaneous B-cell lymphomas - clinicopathological, prognostic and therapeutic characterisation of 54 cases according to the WHO-EORTC classification and the ISCL/EORTC TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sezary syndrome. Leuk Lymphoma 2008;49:1094-103. [Crossref] [PubMed]
- Cerroni L, Signoretti S, Höfler G, et al. Primary cutaneous marginal zone B-cell lymphoma: a recently described entity of low-grade malignant cutaneous B-cell lymphoma. Am J Surg Pathol 1997;21:1307-15. [Crossref] [PubMed]
- Hoefnagel JJ, Vermeer MH, Jansen PM, et al. Primary cutaneous marginal zone B-cell lymphoma: clinical and therapeutic features in 50 cases. Arch Dermatol 2005;141:1139-45. [Crossref] [PubMed]
- Raso T, Bianco O, Grosso B, et al. Achromobacter xylosoxidans respiratory tract infections in cystic fibrosis patients. APMIS 2008;116:837-41. [Crossref] [PubMed]
- Gould SJ, Isaacson PG. Bronchus-associated lymphoid tissue (BALT) in human fetal and infant lung. J Pathol 1993;169:229-34. [Crossref] [PubMed]
- Pabst R. Is BALT a major component of the human lung immune system? Immunol Today 1992;13:119-22. [Crossref] [PubMed]
- Adam P, Czapiewsky P, Colak S, et al. Prevalence of Achromobacter xylosoxidans in pulmonary mucosa-associated lymphoid tissue lymphoma in different regions of Europe. Br J Haematol 2014;164:804-10. [Crossref] [PubMed]
- Aoyama S, Masaki A, Sakamoto Y, et al. Achromobacter infection is rare in Japanese patients with pulmonary B-cell lymphoma. Intern Med 2018;57:789-94. [Crossref] [PubMed]
- Ishimatsu Y, Mukae H, Matsumoto K, et al. Two cases with pulmonary mucosa-associated lymphoid tissue lymphoma successfully treated with clarithromycin. Chest 2010;138:730-3. [Crossref] [PubMed]
- Pervez S, Mumtaz K, Ullah SS, et al. Immunoproliferative small intestinal disease (IPSID). J Coll Physicians Surg Pak 2011;21:57-8. [PubMed]
- Al-Saleem T, Al-Mondhiry H. Immunoproliferative small intestinal disease (IPSID): a model for mature B-cell neoplasms. Blood 2005;105:2274-80. [Crossref] [PubMed]
- Martin IG, Aldoori MI. Immunoproliferative small intestinal disease: Mediterranean lymphoma and alpha heavy chain disease. Br J Surg 1994;81:20-4. [Crossref] [PubMed]
- Rambaud JC, Halphen M, Galian A, et al. Immunoproliferative small intestinal disease (IPSID): relationships with alpha-chain disease and "Mediterranean" lymphomas. Springer Semin Immunopathol 1990;12:239-50. [Crossref] [PubMed]
- Al-Saleem T, Zardawi IM. Primary lymphomas of the small intestine in Iraq: a pathological study of 145 cases. Histopathology 1979;3:89-106. [Crossref] [PubMed]
- Lecuit M, Abachin E, Martin A, et al. Immunoproliferative small intestinal disease associated with Campylobacter jejuni. N Engl J Med 2004;350:239-48. [Crossref] [PubMed]
- Bianchi G, Sohani AR. Heavy chain disease of the small bowel. Curr Gastroenterol Rep 2018;20:3. [Crossref] [PubMed]
- Galian A, Lecestre MJ, Scotto J, et al. Pathological study of alpha chain disease with special emphasis on evolution. Cancer 1977;39:2081-101. [Crossref] [PubMed]
- Salem PA, Nassar VH, Shahid MJ, et al. “Mediterranean abdominal lymphoma” or immunoproliferative small intestinal disease. Part I: Clinical aspects. Cancer 1977;40:2941-7. [Crossref] [PubMed]
- Ben-Ayed F, Halphen M, Najjar T, et al. Treatment of alpha chain disease: results of a prospective study in 21 Tunisian patients by the Tunisian- French intestinal Lymphoma Study Group. Cancer 1989;63:1251-6. [Crossref] [PubMed]
- Akbulut H, Soykan I, Yakaryilmaz F, et al. Five-year results of the treatment of 23 patients with immunoproliferative small intestinal disease: a Turkish experience. Cancer 1997;80:8-14. [Crossref] [PubMed]
- Rambaud J, Halphen M. Immunoproliferative small intestinal disease (IPSID): relationships with alpha-chain disease and “Mediterranean” lymphomas. Gastoenterol Int 1989;2:33-41.
- Salimi M, Spinelli JJ. Chemotherapy of Mediterranean abdominal lymphoma: retrospective comparison of chemotherapy protocols in Iranian patients. Am J Clin Oncol 1996;19:18-22. [Crossref] [PubMed]
- Richards C, Pantanowitz L, Dezube BJ, et al. Antimicrobial and non-antimicrobial tetracyclines in human cancer trials. Pharmacol Res 2011;63:151-6. [Crossref] [PubMed]
- Aoki D, Ueno S, Kubo F, et al. Roxithromycin inhibits angiogenesis of human hepatoma cells in vivo by suppressing VEGF production. Anticancer Res 2005;25:133-8. [PubMed]
- Kurdowska A, Noble JM, Griffith DE. The effect of azithromycin and clarithromycin on ex vivo interleukin-8 (IL-8) release from whole blood and IL-8 production by human alveolar macrophages. J Antimicrob Chemother 2001;47:867-70. [Crossref] [PubMed]
- Yatsunami J, Tsuruta N, Hara N, et al. Inhibition of tumor angiogenesis by roxithromycin, a 14-membered ring macrolide antibiotic. Cancer Lett 1998;131:137-43. [Crossref] [PubMed]
- Ratzinger F, Haslacher H, Poeppl W, et al. Azithromycin suppresses CD4(+) T-cell activation by direct modulation of mTOR activity. Sci Rep 2014;4:7438. [Crossref] [PubMed]
- Niesvizky R, Jayabalan DS, Christos PJ, et al. BiRD (Biaxin [clarithromycin]/Revlimid lenalidomide]/dexamethasone) combination therapy results in high complete- and overall-response rates in treatment-naive symptomatic multiple myeloma. Blood 2008;111:1101-9. [Crossref] [PubMed]
- Coleman M, Leonard J, Lyons L, et al. Treatment of Waldenstrom's macroglobulinemia with clarithromycin, low-dose thalidomide, and dexamethasone. Semin Oncol 2003;30:270-4. [Crossref] [PubMed]
- Govi S, Dognini GP, Licata G, et al. Six-month oral clarithromycin regimen is safe and active in extranodal marginal zone B-cell lymphomas: final results of a single-Centre phase II trial. Br J Haematol 2010;150:226-9. [Crossref] [PubMed]
- Ferreri AJ, Sassone M, Kiesewetter B, et al. High-dose clarithromycin is an active monotherapy for patients with relapsed/refractory extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT): the HD-K phase II trial. Ann Oncol 2015;26:1760-5. [Crossref] [PubMed]
- Ferreri AJM, Cecchetti C, Kiesewetter B, et al. Clarithromycin as a "repurposing drug" against MALT lymphoma. Br J Haematol 2018;182:913-5. [Crossref] [PubMed]
- Wildfeuer A, Laufen H, Zimmermann T. Uptake of azithromycin by various cells and its intracellular activity under in vivo conditions. Antimicrob Agents Chemother 1996;40:75-9. [Crossref] [PubMed]
- Lagler H, Kiesewetter B, Dolak W, et al. Treatment of mucosa associated lymphoid tissue lymphoma with a long-term once-weekly regimen of oral azithromycin: results from the phase II MALT-A trial. Hematol Oncol 2019;37:22-26. [Crossref] [PubMed]
- Hand WL, Hand DL. Characteristics and mechanisms of azithromycin accumulation and efflux in human polymorphonuclear leukocytes. Int J Antimicrob Agents 2001;18:419-25. [Crossref] [PubMed]
- Ballow CH, Amsden GW, Highet VS, et al. Pharmacokinetics of oral azithromycin in serum, urine, polymorphonuclear leucocytes and inflammatory vs non-inflammatory skin blisters in healthy volunteers. Clin Drug Investig 1998;15:159-67. [Crossref] [PubMed]
- Pokorny A, Kiesewetter B, Raderer M, et al. Experience with clarithromycin as antineoplastic therapy for extranodal marginal zone B-cell lymphoma of the mucosa associated lymphoid tissue (MALT-lymphoma) outside of clinical trials: Real-world data from the University of Vienna. Hematol Oncol 2020;38:409-11. [Crossref] [PubMed]
Cite this article as: Sassone M, Ponzoni M, Ferreri AJM. Antibiotic treatment for bacteria-associated extranodal marginal zone lymphoma. Ann Lymphoma 2021;5:4.