Frontline management for follicular lymphoma patients: a narrative review
Introduction
Follicular lymphoma (FL) is the most common indolent non-Hodgkin lymphoma (NHL), accounting for about 70% of all indolent lymphomas and for 20% of NHLs. It is a slow growing disease with an excellent response to treatment, when needed, and with a typical relapsing remitting course associated with a low but significant risk of transformation into an aggressive lymphoma (1).
The availability of very active therapies and the adoption of more accurate diagnostic tools, along with a better understanding of the biology of FL, have determined a meaningful improvement in survival, which is now measured in decades (2).
Despite the fact that the disease is still identified as an incurable neoplasm, the clinical behavior in FL patients is highly heterogeneous. Recently Sarkozy et al., analyzed the cause of death (COD) in a large cohort of FL patients treated in the rituximab era. Overall, at 10 years from diagnosis, the majority of the deaths (57%) were related to lymphoma progression or transformation, with a cumulative incidence of 10.3% at 10 years vs. 3% for non-lymphoma related COD. Of note 10 year cumulative incidence of non-lymphoma related COD was stable across prognostic groups while the risk of lymphoma related COD increased significantly in patients with a high Follicular Lymphoma International Prognostic Index (FLIPI) score (27%), in patients who experienced early events (36%) and in those who transformed into an aggressive lymphoma (46%) (3). This observation suggests that an accurate definition of patient risk should be considered as a key research priority in the field to allow the definition of risk adapted strategies in FL aimed to increase treatment efficacy in high risk patients, and to minimize the toxicity of therapy in the low risk population.
Progression of disease within 24 months from time of treatment start (POD24) allows to identify about 20% of patients at high risk who have only a 50% probability of survival at five years (4-6).
POD24 as well as other factors related to the length of remission (EFS12/24, CR30) are currently identified as strong predictors of poorer OS in FL but cannot be used as predictive factors for treatment naïve patients; they are better seen as early surrogate endpoints for PFS of OS (i.e., CR30) (7-9).
Several prognostic factors and indices have been studied to account for FL patients’ heterogeneous clinical course. Besides the well-established baseline clinical prognostic indices FLIPI and FLIPI2 (10,11) and the more recent PRIMA-PI index (Table 1) (12), the use of clinical genetic risk scores (13,14) has been proposed as useful method to predict patient outcomes and to identify high risk subjects. None of the above-mentioned prognostic features has yet been translated into a predictive tool to guide treatment choices and to design and deliver a personalized approach to FL patient care.

Full table
Recent data demonstrated that survival can be prognosticated also by evaluating response to first-line treatment either using FDG-PET or applying molecular biology techniques to define metabolic or molecular response, respectively (15-18).
A pooled analysis of postinduction FDG-PET in three studies, the PRIMA, PET Folliculaire (PET-FL), and FOLL05 study, confirmed the primary role of metabolic response (MR) defined applying standardized response criteria to end of induction FDG-PET (the international 5-point scale 5PS, or Deauville Score, DS) (19). More recently, data from the large prospective GALLIUM study confirmed the predictive power of MR (DS cut-off ≥4) after either Rituximab- or Obinutuzumab-chemotherapy [Bendamustine, cyclophosphamide, hydroxydaunorubicin, oncovin, and prednisone (CHOP) or Cyclophosphamide, Vincristine, and Prednisone (CVP)]. In this analysis post-induction FDG-PET/CT was prognostic for both PFS and OS (Complete MR (CMR) vs. non-CMR: HR: 0.2, 95% CI: 0.1–0.3; P<0.0001 and HR: 0.2, 95% CI: 0.1–0.5; P<0.0001, respectively) (20).
Regarding molecular response, the presence of t(14;18) chromosomal translocation and of clonal rearrangement of Immunoglobulin genes in FL cells makes it feasible to use high sensitivity techniques to detect the disease in peripheral blood and bone marrow sample and to study minimal residual disease (MRD). Several studies demonstrated that the absence in the bone marrow and peripheral blood of neoplastic cells bearing the bcl-2/IgH rearrangement after treatment is strongly associated with a reduced risk of recurrence (21-25). The correlation between molecular response and survival was also confirmed in the GALLIUM trial, with persistently improved PFS seen in the MRD-negative patients (n=564) compared with the MRD-positive patients (n=70; HR: 0.38; CI: 0.26–05.6; P≤0.0001) (20). Although promising the routine use of MRD in clinical practice, is still limited by lack of consensus and standardization on MRD techniques and timing and by the lack of a molecular marker in all patients with FL. Technical developments, such as the use of novel molecular markers or the adoption of high throughput molecular techniques are gaining momentum in the assessment of molecular response of FL and will likely contribute to transfer MRD analysis to daily clinical practice in the future (26).
In summary prognostic research is very active in FL to validate accurate tools that could clearly identify patients at different risk and to support the development of risk adapted strategies. Among studied factors only those describing the quality of response received enough validation and were stable enough with different therapies to allow the design of response adapted treatments (27,28).
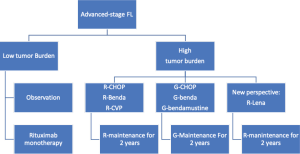
Currently available treatment algorithms define the initial approach to FL patients based on the clinical stage, tumor burden, and symptoms (Figure 1). Patients are usually categorized into those with localized or with advanced stage; the latter group is further divided into low or high tumor burden based on the presence of specific criteria (i.e., GELF, Table 2). Several alternative options are available for these different groups, ranging from observation to radiotherapy (RT) or immunochemotherapy. While treatment decision is straightforward in many patients with FL, it can be challenging in some cases due to the availability of alternative options that are not well supported by adequate evidence. Indeed, none of the recent available clinical trials conducted to improve treatment efficacy was able to modify the overall survival (OS) of patients, thus justifying different approaches for similar conditions. Further, the long natural history of FL and the possibility of achieving good control of the disease even in relapsed patients makes analysis of OS difficult to manage. Earlier endpoints such as progression-free survival (PFS) and treatment tolerability are therefore more adequate than OS to assess the risk-benefit ratio in different clinical settings.

Full table
In this review we discussed some of the most relevant clinical questions that need to be answered to treat naïve FL patients and to summarize the recent evidence on upfront therapy. Given both the indolent nature of the disease and the risk of relapse, a discussion about FL treatment cannot be limited only to the choice of first-line therapy; it should be considered in terms of a more complex therapeutic strategy that also accounts for subsequent relapses of the disease.
We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/aol-20-51).
Management of early-stage disease
Early-stage FL, which includes Ann-Arbor stages I and II, represents about 10–15% of all cases (32). The administration of local RT offers a very good chance of disease control for these patients (2,32). In the FDGPET era, stage I patients have high response rates, and more than two-thirds of patients remain in remission at 5 years from diagnosis, with most relapses occurring at sites distant to the irradiated field (33,34). A retrospective analysis of 512 patients conducted by the International Lymphoma Radiation Oncology Group (ILROG) (33) found that also stage II patients, whose risk of progression remained at 50%, had less chance of cure but excellent control of disease. Very recently the results of the long term follow up from British Columbia population bases analysis confirmed the curative potential of RT alone for limited-stage FL with almost 50% of patients remaining free from progression at 15 years (35).
Regarding RT dose, this was historically fixed at 36–40 Gy on involved sites. Subsequently, Lowry et al. randomly compared 40–45 Gy in 20–23 fractions with 24 Gy in 12 fractions in a large cohort of indolent lymphoma patients, including 185 with FL. With a median follow-up of 5.6 years, the authors concluded that doses of RT can safely be reduced to 24 Gy while still achieving good control of the disease, without any differences in terms of PFS or OS between low and high doses (for PFS, HR:1.06; 95% CI: 0.83–1.34, and for OS, HR:0.96; 95% CI: 0.66–1.41). As expected, there was a trend for reduced acute and late toxicities in the lower-dose arm. From that point on, this low radiation dose has become the new standard (36).
The results of the FORT trial, which compared RT delivered at 4 Gy in two fractions with a standard dose of 24 Gy in 12 fractions (548 patients enrolled, 86% with FL), confirmed the higher dose as the standard of care (HR: 3.42; 95% CI: 2.09–5.55, P<0.0001). Despite the fact that this trial did not meet its primary endpoint, the 4 Gy dose level showed activity in almost 75% of patients, with more than 40% of complete remission (CR). This low-dose approach might currently be used to minimize the side effects in particular sites (parotid glands or lacrimal gland) or with a palliative intent (37).
To improve disease control in early stages of FL, and given that FL is a systemic disease, efforts have been made to further reduce the risk of relapse by evaluating the addition of immunotherapy or of immunochemotherapy to RT.
Combining Rituximab (R) with RT has shown promising outcomes, although the quality of the evidence has been low due to the retrospective nature of the studies and the lack of randomized controlled trials (38-42).
The TROG 99.03 phase III randomized trial compared the efficacy of immunochemotherapy vs. observation after involved-field RT delivered at 36 Gy in 150 stage I-II FL patients. The primary endpoint of this trial was PFS; treatment consisted of a combination of CVP, with or without rituximab. The study showed better 5- and 10-year PFS in the combination arm (59% and 41%, respectively; HR: 0.57; 95% CI: 0.33–0.95, P=0.033). Although the primary objective of the study was achieved, this trial has two criticisms that reduce the transferability of its result to clinical practice: first, rituximab was combined with CVP only in a fraction of the patients; second, no difference in term of OS was observed between arms, thus confirming that delaying immunochemotherapy after RT is at least as effective as immediate treatment, although associated with a higher risk of relapse (43).
In conclusion, local RT remains the approach to early-stage FL patients with the best risk-benefit profile. Adding more therapy to radiation results in overtreatment for most patients, with the added risk of inducing treatment-related toxicity and chemoresistance to the disease. In selected cases for whom it is necessary to avoid the side effects of radiation (i.e., based on the location of the disease) or if the patient refuses local therapy, or in stage II patients with non-contiguous lesions, a better alternative to RT or combined modality treatment is to simply follow the patients, without prescribing any treatment.
Do all patients need treatment?
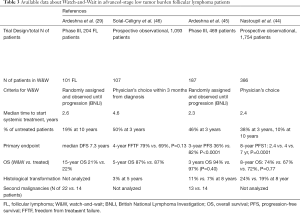
FL is characterized by the appearance of slow growing masses that are rarely associated with systemic symptoms in the early phases of the disease. Moreover, considering that spontaneous regression has been reported in up to 10% of cases, a reasonable approach to asymptomatic patients is to adopt an expectant management. The concept of watchful waiting i.e., not start any treatment until the disease has become symptomatic, was first introduced in the pre-rituximab era and was suggested as the standard approach to advanced stage patients. In the pivotal trial conducted by Ardeshna et al., survival in patients without symptoms, who were only followed, without any treatment, was not lower than that in patients who were immediately treated. Further, the former group had a 30% probability of remaining treatment free for more than 3 years after diagnosis (29). Since then, more effective therapies have become available, with a considerable improvement in the outcomes of treated patients. Nastoupil et al., in a large prospective National LymphoCare Study enrolling 1,754 patients, compared the outcome of patients with stage II-IV FL initially referred to a watch-and-wait (W&W) strategy (n=386) with the outcomes of those treated with rituximab (n=296) or with immunochemotherapy (n=1,072). The long-term follow-up analysis showed that, while improvement in the time to next treatment (TTNT) and in PFS were associated with the use of systemic therapy, there was no effect on OS among the 3 treatment groups, thus confirming the W&W strategy as a reasonable option even in the rituximab era (44). Ardeshna et al. compared W&W with rituximab monotherapy in 379 patients enrolled in a randomized phase III trial and reached similar conclusions, i.e., that delaying treatment start in asymptomatic subjects was associated with higher risk of FL progression but did not translate into any change in patient survival (45).
In addition to main survival outcomes, W&W strategy was not associated with a higher risk of transformation compared with immediate treatment (Table 3) (34,46-48).
Full table
A key aspect of the initial approach to patients with asymptomatic advanced-stage FL is to adopt objective, validated criteria to safely delay treatment start. Among the criteria available, those defined by the Groupe d’Etude des Lymphomes Folliculaires (GELF) help define the tumor burden of FL and are widely used in clinical practice. The GELF criteria define tumor burden as high if any of the following criteria are present: nodal or extranodal masses larger than 7 cm; more than 3 nodal sites involved with a diameter of >3 cm; the presence of B symptoms; serum effusion or organ compression; substantial splenomegaly; cytopenia and elevated serum levels of LDH; level of beta2-microglobuline (30). Similar to GELF, other criteria have been proposed (e.g., BNLI, NCCN) (29,31) but they have been less extensively used. All the criteria available have slight differences in the definition of tumor burden and are mostly based on clinical and laboratory assessment (Table 1). The future development of criteria to define tumor burden in FL should also provide guidance on how to interpret pathological features of the disease (i.e., histology grading, proliferation index) and some of the promising metabolic parameters defined with FDG-PET (i.e., metabolic tumor volume, SUVmax or SUV range) (49-52).
In conclusion, in patients with advanced-stage FL with low tumor burden, W&W remains a reasonable option that is not detrimental to the patient. The decision about whether to delay immediate therapy should always be based on the use of objective criteria and should be carefully explained and discussed with the patient as the safest option to manage the disease without adding any additional risk. Single agent rituximab remains the best and more frequently alternative option to W&W in low tumor burden FL (45). In these cases, a short course of rituximab with retreatment when required was shown to be equally effective but less toxic and less expensive when compared to a full induction treatment with rituximab followed by 2 year maintenance with the same agent (47).
Which options are appropriate if systemic therapy is required?
The scientific community has been debating for decades what the best choice is among the various chemotherapy regimens available for the initial treatment of patients with advanced-stage high tumor burden FL. The discussion was only temporarily suspended in the early 2000s, when the anti-CD20 monoclonal antibody rituximab became available and immunochemotherapy (ICT), the combination of rituximab with one or more chemotherapy agents, was identified as the undisputed standard for treating symptomatic patients with FL (53-58). Soon after ICT was identified as standard therapy and the old questions about the choice of which chemotherapy add to rituximab came back as urgent unmet need. In particular, the debate has been centered on the efficacy of anthracycline-containing regimens for many years; recently, the availability of the novel chemotherapy agent bendamustine has also been debated.
The Italian randomized trial FOLL05 was conducted to compare the efficacy of the anthracycline-containing regimen, CHOP, and of the use of the purine analogue fludarabine combined with mitoxantrone (FM) with a reference treatment with CVP, all combined with rituximab. The study was first published in 2012, with the update published in 2018. Both early and mature results of the trial confirmed R-CHOP and R-FM as better regimens than R-CVP in terms of PFS (HR for PFS adjusted by FLIPI2 versus R-CVP was 0.73 for R-CHOP (95% CI: 0.54–0.98; P=0.037) and 0.67 for R-FM (95% CI: 0.50–0.91; P=0.009) but also showed higher rates of acute and late toxic events for patients treated with R-FM compared to R-CHOP. Interestingly, the trial was not able to show any difference among study treatments in terms of OS, thus confirming R-CHOP as the best option for most patients, and R-CVP as a suitable choice for older patients or for patients who are not good candidates to receive anthracyclines (57,59).
The efficacy of bendamustine in combination with rituximab was analyzed by two randomized trials and compared with that of R-CHOP or R-CVP. The first study, by the German Stil group, showed better efficacy of R-Bendamustine (R-B) vs. R-CHOP in 513 patients with advanced stage treatment-naïve indolent lymphoma, including a subset of 279 FL patients. R-B resulted in a better PFS (HR: 0.58, PFS 69 vs. 31 months, respectively, P<0.0001) and significantly reduced the risk of toxicity, grade III-IV neutropenia (29% vs. 69% in favor of RB), alopecia, peripheral neuropathy, and stomatitis compared with R-CHOP. Regarding OS, the 10-year follow-up did not highlight statistically significant differences between the two different arms (10-year OS 71% vs. 66% respectively) (56).
The second study, known as the BRIGHT trial, compared the R-B regimen with R-CHOP or R-CVP combination in previously untreated indolent and Mantle cell lymphomas in 314 FL patients. The study met the primary endpoint, which was to show non-inferiority of R-B vs. R-CHOP/CVP in terms of CR rates, (CR was 31% vs. 25% in the R-CHOP/CVP and R-B arm, respectively) (58). The long term follow up of the study, recently updated, confirmed the better PFS rate at 5 years for BR over R-CHOP/R-CVP (65.6% vs. 55.8%, P=0.0025). However, no significant difference in OS was observed and for the safety profile, a higher number of secondary malignancies was noted in the BR treatment group (60).
Currently, a simple trial conducted with a head-to-head comparison of R-B and R-CHOP regimens on a well-sized population of FL patients is lacking, and long-term analyses of the available trials have not been able to show any difference in terms of OS between treatments. Moreover, a direct comparison of the two regimens in specific subsets of patients, including cases with an aggressive pathology profile (i.e., Grade 3a, high proliferation index, high SUV at FDG-PET) or older patients, is not available. As shown by the characteristics of patients treated with CHOP or bendamustine in the Gallium trial, patients treated with CHOP were more frequently characterized by worse clinical and prognostic features: 47% were in the FLIPI high-risk group (vs. 40% in the bendamustine and 35% in the CVP groups) and more frequently had a bulky disease (52% vs. 40% vs. 40%, respectively). Patients treated with bendamustine were older than those treated with CHOP and presented with a major grade of frailty [24% with Charlson Comorbidity Index CCI score >1 vs. 17% (CHOP) and 19% (CVP)] (61). These differences in patient characteristics reflect the decision of the treating physician and correspond to a perceived, but not clearly demonstrated, better profile of CHOP for the treatment of young high-risk patients, leaving the use of bendamustine to all other subjects (61,62).
In conclusion, for the frontline therapy of advanced-stage symptomatic FL, several chemotherapy options are available, all of which can be combined with rituximab; none has ever been associated with an OS advantage. Among the available options, bendamustine has the highest consensus, despite the fact that some concerns regarding the risk of immunodepression and of late events are still debated. Treatment choice should therefore be based on several factors including, but not limited to, patient age, the presence of comorbidities, the prognostic profile of the disease, and the patient’s preferences.
Is maintenance with anti-CD20 Moab required?
The natural history of FL is characterized by frequent relapses and by the progressively reduced possibility of achieving deep, prolonged response after each recurrence, thus prompting clinical researchers to identify post-induction maintenance therapies (MT) as a promising strategy to prolong the duration of response and to reduce the risk of progression. MT was first developed using interferon, with promising results, but the success of MT was clearly achieved with anti-CD20 monoclonal antibodies and was first demonstrated in the relapsed refractory and in the frontline settings (47,63,64).
The PRIMA study provided randomized evidence that MT with rituximab could prolong PFS in 1,217 patients responding to frontline R-CHOP, RCVP, or RFCM. The extended follow-up (median 9 years) confirmed the initial results, with a 50% reduction in the risk of progression for the MT group compared to observation (10.5 vs. 4 years median PFS, respectively, HR: 0.61; P<0.0001). Although better PFS was seen across all categories, including age, sex, FLIPI score, and in patients achieving both complete response (CR) and partial response (PR), no difference has yet emerged in terms of OS (80% at 10 years in both treatment arms). Conversely, as expected, an increased risk of infections has been observed for MT (65-67).
Based on the results of the PRIMA trial, MT with rituximab has been identified as the recommended strategy for patients responding to ICT, and MT has been included as part of induction therapy in recent clinical trials and in novel treatment recommendations (62). Although the efficacy of MT is suggested after all available ICT regimens, any formal confirmation of the efficacy of MT with schemes other than R-CHOP/R-CVP is not currently available. This observation mainly applies to the use of MT in patients treated with RB regimen (68-70).
With the availability of different accurate prognostic tools, and with the lack of MT effects on OS, an important question is whether chemoimmunotherapy followed by MT is really needed for all patients with FL.
The concept of treatment adaptation in FL has not yet been extensively studied, but the FOLL12 trial by the Fondazione Italiana Linfomi (NCT02063685) was the first to use a simple feature like the quality of response to induction ICT as a predictive factor (7-9,38,55-59). This trial enrolled treatment-naïve FL patients who were randomized to receive either a standard therapy of ICT followed by MT or a response-adapted strategy that used metabolic and molecular response to define post-induction management. Patients with complete MR were only observed and treated with rituximab if they turned positive at molecular evaluation [t(14;18) used as molecular marker]; instead, patients with partial MR received one dose of radioimmunotherapy prior to starting standard MT with rituximab. The study was initially designed to confirm the non-inferiority of the two strategies in terms of PFS, but from the preliminary results on the 802 randomized patients, the reference arm showed clearly better results than did the response-adapted arm. When interpreting the preliminary FOLL12 results, the main comment concerns the very high activity of currently available ICT, which was confirmed by the observed 88% CR rate and which reduced the accuracy of metabolic assessment of response in predicting the risk of relapse (27). Like the FOLL12 trial, the Petrea trial, which is currently enrolling patients in the UK and Australia, will try to assess the efficacy of a slightly different response-adapted approach in FL (28).
Which anti-CD20 monoclonal antibody should be combined with chemotherapy?
Of the second generation anti-CD20 monoclonal antibodies, obinutuzumab (also known as GA101- G) is a humanized type II glycoengineered antibody targeting CD20 (71) that has shown superior efficacy as compared to rituximab, inducing direct cell death and enhanced antibody-dependent cellular cytotoxicity (with less complement-dependent cytotoxicity) (72-76).
The efficacy of the frontline use of the combination of obinutuzumab with chemotherapy, followed by obinutuzumab maintenance, was compared with standard rituximab-based immunochemotherapy plus rituximab maintenance in the large phase III randomized Gallium trial. The study enrolled 1,202 patients and was sized to show an improvement in PFS for patients randomized to the obinutuzumab arm. The most recent data, released after a median follow-up of 73months, confirmed the initial results, which showed that the use of obinutuzumab resulted in a significant improvement both in PFS and in time to next anti-lymphoma treatment (TTNT), with a reduction in the risk of progression of approximately 30% for the obinutuzumab arm compared to the rituximab arm (The PFS rate was 78.1% vs. 67.2%, respectively, for G vs. R, HR: 0.73; 95% CI: 0.59–0.90; P=0.03) and the 4-year TTNT was 84% for obinutuzumab arm vs. 76.7% in rituximab arm).
Of note, even though no difference between the study arms in terms of OS was observed, the use of obinutuzumab was also associated with a 44% reduction in the risk of disease progression within 24 months (POD24) (12.5% vs. 18.9% in the G vs. R arms, respectively) (9).
Preplanned subgroup analyses of chemotherapy regimen and of FLIPI risk groups revealed that G-based ICT was more effective than R-based ICT, the only exception being in low-risk FLIPI subjects (0–1 risk factors) (9,62). The greater efficacy of obinutuzumab was also observed for all the chemotherapy regimens used in the trial, including CHOP, CVP, or bendamustine.
Regarding the Gallium trial’s safety profile analysis, patients who received obinutuzumab were more likely to experience grade >3 adverse events and serious adverse events than those treated with rituximab (79% vs. 71% and 48% vs. 42%, respectively). In detail, higher rates of neutropenia and infection rates with G [Grades 3 to 5 adverse events (74.6% vs. 67.8%) and serious adverse events (46.1% vs. 39.9%)].
Of note a post hoc analysis of the GALLIUM trial showed that patients receiving bendamustine induction had a higher number of acute and late toxicities, regardless of the monoclonal antibody used (61).
The Gallium results, and in particular the estimated 80% rate of PFS at 3 years for patients treated with obinutuzumab, are the best ever achieved with immunochemotherapy in the frontline treatment of advanced-stage high tumor burden patients, thereby prompting several national health systems to approve the use of the drug in all patients, with the exclusion of low-risk subjects in some countries. The lack of a benefit of G vs. R in terms of OS and the higher frequency of adverse events associated with the former, in particular when combined with bendamustine, leave the choice of whether to use systemic immunochemotherapy to the treating physician (3).
Will chemotherapy-free treatment replace immunochemotherapy?
FL is an excellent disease model to develop and evaluate therapeutic approaches that do not require the use of chemotherapeutic agents due to its long natural history and to its high sensitivity to chemotherapy-free agents, including anti-CD20 monoclonal antibodies and new targeted agents.
The activity of single-agent rituximab in patients with limited stage and low tumor burden FL has already been discussed. The first study to assess the efficacy of treating advanced-stage high tumor burden FL with a chemotherapy-free approach were conducted by Ghielmini et al., who compared short vs. prolonged use of rituximab (4 vs. 8 doses) in treatment-naïve and in relapsed refractory patients (62,77). At a median follow-up of 9.5 years, the study showed that the median event-free survival (EFS) was 13 months for the short and 24 months for the prolonged exposure groups (P<0.001). Of previously untreated patients receiving maintenance, 45% were still without any events at 8 years. No long-term toxicity potentially due to rituximab was observed (69,70). The rituximab-based chemotherapy-free approach was further studied in a subsequent randomized study by the SAKK and the Nordic group, which compared 8 doses of rituximab with a longer treatment with the same agent for up to 5 years (78-80). This second trial was not able to demonstrate the superiority of the prolonged use of rituximab in terms of the primary study endpoint (EFS), but it did confirm that for the 40% of patients who responded to an initial treatment with rituximab, chemotherapy-free treatment was associated with an excellent median PFS (7.4 vs. 3.5 years, respectively; P=0.04).
Rituximab monotherapy has never been compared with immunochemotherapy treatment, but in a retrospective analysis by the Nordic group, the 10- and 15-year OS rate for FL patients initially treated with rituximab after random assignment was 75% and 65%, without any difference compared to immunochemotherapy (81).
Deeper insight into the biological landscape of the disease has laid the foundation for the identification of new chemotherapy-free agents targeting intracellular pathways, the cell surface, and the microenvironment. In particular, knowledge of a distinctive tumor microenvironment and related immune dysfunction associated with FL has contributed to the promotion of novel combinations, such as rituximab plus lenalidomide (R2) (82-84).
Two randomized trials have identified R2 as a promising treatment and have compared it with R monotherapy (SAKK35-10) or with immunochemotherapy. In the first, a phase II randomized trial, 154 untreated FL patients in need of therapy were randomly assigned to rituximab or rituximab plus lenalidomide. After a median follow-up of 4 years, the combination arm showed better control of the disease, with a significantly higher 30-month CR/Cru (42% vs. 19%), and longer PFS and TTNT. The OS rate was excellent in both arms (more than 90%), with more frequent grade 3–4 adverse events in the lenalidomide arm, as expected (85).
The Relevance study was designed as a randomized phase III trial to demonstrate the greater efficacy of R2 as a frontline treatment compared with standard ICT. The study enrolled 1030 previously untreated FL patients in need of treatment according to the GELF criteria. The study was not able to demonstrate superior efficacy of R2 over standard chemoimmunotherapy as it failed to meet the primary endpoint of superior CR and PFS. At a median follow-up of 37.9 months, the interim 3-year rate of PFS was 77% (95% CI: 72–80%) and 78% (95% CI: 74–82%), for R2 and R-Chemo, respectively. As expected, the two groups had different safety profiles: a higher percentage of patients in the rituximab-chemotherapy group had grade 3 or 4 neutropenia (32% vs. 50%) and febrile neutropenia of any grade (2% vs. 7%), and a higher percentage of patients in the rituximab-lenalidomide group had grade 3 or 4 cutaneous reactions (7% vs. 1%) (64).
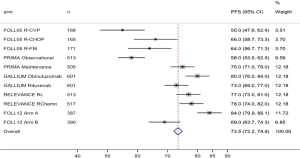
The efficacy results are consistent with 3-year rates of PFS of 73% and 75% in the robust, previously reported results of the Gallium and PRIMA trials. Moreover, a similar rate of histologic transformation was observed in the two cohorts, less than 1% per year (59).
In addition, in a very recent subanalysis of the Relevance trial, R2 showed a high rate of molecular response that was similar to that of R-Chemo after only 6 months of treatment (86).
This similar efficacy and favorable tolerability make the R2 regimen a potential new approach to treating FL in the first-line setting for some patients, especially those wishing to avoid hematologic toxicity. Recently Nastoupil et al., conducted a phase II study in 90 previously untreated high tumor burden FL patients who received a combination of Obinutuzumab and Lenalidomide for a total of 6 cycles. With a median follow-up of 25 months, 87% achieved a CR as first response and the estimated 2-year PFS was 96%. This novel combination had a favourable and manageable toxicity profile similar to that observed for R2. If these results will be confirmed with more patients and longer follow up the combination of Obinutuzumab and lenalidomide might become a promising option to further develop a chemo-free management of treatment naïve FL (87).
In conclusion, there is a strong argument in favor of opting for chemotherapy-free management in FL, including for previously untreated patients. Available chemotherapy-free options are mainly based on the use of rituximab alone or in combination with lenalidomide; currently, there is a suggestion that chemotherapy-free treatment has similar efficacy compared to the ICT available. Several questions still need to be answered to modify the current approach to previously untreated FL patients, regarding long-term results, salvageability of relapsed patients, and the activity of the chemotherapy-free option in specific groups of patients, including but not limited to young vs. old patients and histologic grade 1–2 vs. 3a.
Conclusions
In conclusion, FL is a complex neoplasm of the immune system, with a long natural history and a high sensitivity to the several available treatments. The choice of front-line treatment in FL is straightforward in many patients. Nevertheless, it is always a critical decision as physicians should carefully balance the intensity of the therapeutic intervention with the patient’s real need of therapy. Despite the fact that FL is still considered an incurable disease, a growing proportion of patients are expected to receive only one line of therapy in their lifetime, as time to progression can be long enough to compete with the natural course of life. In the context of relapsing patients requiring additional lines of therapy, the choice of initial treatment is equally relevant due to the possibility of acting on additional events related to the lymphoma (i.e., histologic transformation, chemoresistance) or related to the patient (chronic immunosuppression, cardiac toxicity, organ disfunction), thereby granting the patients the best chance of a long life. In all cases therapeutic decision in FL should always be based on a strong knowledge of the biology and pathology of the disease, about available therapies, and about the patient’s medical and social conditions and personal wishes.
More therapeutic options for the management of FL are expected in the next few years. However, given the current general approaches to FL patients (Figure 1), further improvement in survival endpoints is unlikely for the first-lines of therapy, considering the excellent results achieved with the most recent studies (46,62) in terms of disease control and survival (Figure 2). In this context clinical research should start to focus on additional endpoints that are relevant for a disease with a long natural history and should include the long-term safety profile of therapy and patients’ perspective. Moreover, it is time to move FL into the field of personalized medicine. To do so, reliable prognostic tools should be identified and validated and be accurate enough to identify the different needs of each patient at the time of diagnosis.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Mark Roschewski, Carla Casulo) for the series “Follicular Lymphoma” published in Annals of Lymphoma. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/aol-20-51
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aol-20-51). The series “Follicular Lymphoma” was commissioned by the editorial office without any funding or sponsorship. SL reports that in the previous three years he acted as advisor for the following companies: Roche, BMS, Gilead, Genmab, Jannsen, and Regeneron. VT has no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zelenetz AD, Gordon LI, Wierda WG, et al. Non-Hodgkin’s lymphomas, version 4.2014. J Natl Compr Canc Netw 2014;12:1282-303. [Crossref] [PubMed]
- Tan D, Horning SJ, Hoppe RT, et al. Improvements in observed and relative survival in follicular grade 1-2 lymphoma during 4 decades: the Stanford University experience. Blood 2013;122:981-7. [Crossref] [PubMed]
- Sarkozy C, Maurer MJ, Link BK, et al. Cause of Death in Follicular Lymphoma in the First Decade of the Rituximab Era: A Pooled Analysis of French and US Cohorts. J Clin Oncol 2019;37:144-52. [Crossref] [PubMed]
- Launonen A, Hiddemann W, Duenzinger U, et al. Early Disease Progression Predicts Poorer Survival in Patients with Follicular Lymphoma (FL) in the GALLIUM Study. Blood 2017;130:1490.
- Casulo C, Byrtek M, Dawson KL, et al. Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: An analysis from the National LymphoCare Study. J Clin Oncol 2015;33:2516. [Crossref] [PubMed]
- Casulo C, Le-Rademacher J, Dixon J, et al. Validation of POD24 As a Robust Early Clinical Endpoint of Poor Survival in Follicular Lymphoma: Results from the Follicular Lymphoma Analysis of Surrogacy Hypothesis (FLASH) Investigation Using Individual Data from 5,453 Patients on 13 Clinical Trials. Blood 2017;130:412.
- Shi Q, Flowers CR, Hiddemann W, et al. Thirty-Month Complete Response as a Surrogate End Point in First-Line Follicular Lymphoma Therapy: An Individual Patient-Level Analysis of Multiple Randomized Trials. J Clin Oncol 2017;35:552-60. [Crossref] [PubMed]
- Maurer MJ, Ghesquières H, Jais JP, et al. Event-Free Survival at 24 Months Is a Robust End Point for Disease-Related Outcome in Diffuse Large B-Cell Lymphoma Treated With Immunochemotherapy. J Clin Oncol 2014;32:1066-73. [Crossref] [PubMed]
- Seymour JF, Marcus R, Davies A, et al. Association of early disease progression and very poor survival in the GALLIUM study in follicular lymphoma: benefit of obinutuzumab in reducing the rate of early progression. Haematologica 2019;104:1202-8. Erratum in: Haematologica 2020;105:1465. [Crossref] [PubMed]
- Solal-Céligny P, Roy P, Colombat P, et al. Follicular lymphoma international prognostic index. Blood 2004;104:1258-65. [Crossref] [PubMed]
- Federico M, Bellei M, Marcheselli L, et al. Follicular lymphoma international prognostic index 2: a new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. J Clin Oncol 2009;27:4555-62. [Crossref] [PubMed]
- Bachy E, Maurer MJ, Habermann TM, et al. A simplified scoring system in de novo follicular lymphoma treated initially with immunochemotherapy. Blood 2018;132:49-58. [Crossref] [PubMed]
- Pastore A, Jurinovic V, Kridel R, et al. Integration of gene mutations in risk prognostication for patients receiving first-line immunochemotherapy for follicular lymphoma: a retrospective analysis of a prospective clinical trial and validation in a population-based registry. Lancet Oncol 2015;16:1111-22. [Crossref] [PubMed]
- Huet S, Tesson B, Jais JP, et al. A gene-expression profiling score for prediction of outcome in patients with follicular lymphoma: a retrospective training and validation analysis in three international cohorts. Lancet Oncol 2018;19:549-61. [Crossref] [PubMed]
- Luminari S, Biasoli I, Versari A, Rattotti S, et al. The prognostic role of post-induction FDG-PET in patients with follicular lymphoma: a subset analysis from the FOLL05 trial of the Fondazione Italiana Linfomi (FIL). Ann Oncol 2014;25:442-7. [Crossref] [PubMed]
- Dupuis J, Berriolo-Riedinger A, Julian A, et al. Impact of [(18)F]fluorodeoxyglucose positron emission tomography response evaluation in patients with high-tumor burden follicular lymphoma treated with immunochemotherapy: a prospective study from the Groupe d’Etudes des Lymphomes de l’Adulte and GOELAMS. J Clin Oncol 2012;30:4317-22. [Crossref] [PubMed]
- Trotman J, Fournier M, Lamy T, et al. Positron emission tomography-computed tomography (PET-CT) after induction therapy is highly predictive of patient outcome in follicular lymphoma: analysis of PET-CT in a subset of PRIMA trial participants. J Clin Oncol 2011;29:3194-200. [Crossref] [PubMed]
- Luminari S, Galimberti S, Versari A, et al. Positron emission tomography response and minimal residual disease impact on progression-free survival in patients with follicular lymphoma. A subset analysis from the FOLL05 trial of the Fondazione Italiana Linfomi. Haematologica 2016;101:e66-8. [Crossref] [PubMed]
- Trotman J, Luminari S, Boussetta S, et al. Prognostic value of PET-CT after first-line therapy in patients with follicular lymphoma: a pooled analysis of central scan review in three multicentre studies. Lancet Haematol 2014;1:e17-27. [Crossref] [PubMed]
- Trotman J, Davies A, Hiddemann W. Relationship between MRD and PET responses and PFS in previously untreated follicular lymphoma in the GALLIUM trial. J Clin Oncol 2018;36:7557. [Crossref]
- Corradini P, Astolfi M, Cherasco C, et al. Molecular monitoring of minimal residual disease in follicular and mantle cell non-Hodgkin’s lymphomas treated with high-dose chemotherapy and peripheral blood progenitor cell autografting. Blood 1997;89:724-31. [Crossref] [PubMed]
- Hardingham JE, Kotasek D, Sage RE, et al. Significance of molecular marker-positive cells after autologous peripheral-blood stem-cell transplantation for non-Hodgkin’s lymphoma. J Clin Oncol 1995;13:1073-9. [Crossref] [PubMed]
- Rambaldi A, Lazzari M, Manzoni C, et al. Monitoring of minimal residual disease after CHOP and rituximab in previously untreated patients with follicular lymphoma. Blood 2002;99:856-62. [Crossref] [PubMed]
- Galimberti S, Luminari S, Ciabatti E, et al. Minimal residual disease after conventional treatment significantly impacts on progression-free survival of patients with follicular lymphoma: The FIL FOLL05 trial. Clin Cancer Res 2014;20:6398-405. [Crossref] [PubMed]
- Ladetto M, Lobetti-Bodoni C, Mantoan B, et al. Persistence of minimal residual disease in bone marrow predicts outcome in follicular lymphomas treated with a rituximab-intensive program. Blood 2013;122:3759-66. [Crossref] [PubMed]
- Grimaldi D, Genuardi E, Ferrante M, et al. Minimal Residual Disease in Indolent Lymphomas: A Critical Assessment. Curr Treat Options Oncol 2018;19:71. [Crossref] [PubMed]
- Federico M, Mannina D, Versari A, et al. Response oriented maintenance therapy in advanced follicular lymphoma. Results of the interim analysis of the foll12 trial conducted by the FONDAZIONE ITALIANA LINFOMI. Hematol Oncol 2019;37:153-4. [Crossref]
- Pettitt AR, Barrington S, Kalakonda N, et al. Ncri petrea trial: a phase 3 evaluation of pet-guided, response-adapted therapy in patients with previously untreated, advanced-stage, high-tumour-burden follicular lymphoma. Hematol Oncol 2019;37:67-8. [Crossref]
- Ardeshna KM, Smith P, Norton A, et al. Long-term effect of a watch and wait policy versus immediate systemic treatment for asymptomatic advanced-stage non-Hodgkin lymphoma: a randomised controlled trial. Lancet 2003;362:516-22. [Crossref] [PubMed]
- Brice P, Bastion Y, Lepage E, et al. Comparison in low-tumor-burden follicular lymphomas between an initial no-treatment policy, prednimustine, or interferon alfa: a randomized study from the Groupe d’Etude des Lymphomes Folliculaires. Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol 1997;15:1110-7. [Crossref] [PubMed]
- Zelenetz AD, Gordon LI, Abramson JS, et al. NCCN Guidelines Insights: B-Cell Lymphomas, Version 3.2019. J Natl Compr Canc Netw 2019;17:650-61. [Crossref] [PubMed]
- Freedman A, Jacobsen E. Follicular lymphoma: 2020 update on diagnosis and management. Am J Hematol 2020;95:316-27. [Crossref] [PubMed]
- Brady JL, Binkley MS, Hajj C, et al. Definitive radiotherapy for localized follicular lymphoma staged by 18F-FDG PET-CT: a collaborative study by ILROG. Blood 2019;133:237-45. [Crossref] [PubMed]
- Eich HT, Heimann M, Stützer H, et al. Long-term outcome and prognostic factors in early-stage nodal low-grade non-hodgkin’s lymphomas treated with radiation therapy. Strahlenther Onkol 2009;185:288-95. [Crossref] [PubMed]
- Lo AC, Campbell BA, Pickles T, et al. Long-term outcomes for patients with limited-stage follicular lymphoma: update of a population-based study. Blood 2020;136:1006-10. [Crossref] [PubMed]
- Lowry L, Smith P, Qian W, et al. Reduced dose radiotherapy for local control in non-Hodgkin lymphoma: a randomised phase III trial. Radiother Oncol 2011;100:86-92. [Crossref] [PubMed]
- Hoskin PJ, Kirkwood AA, Popova B, et al. 4 Gy versus 24 Gy radiotherapy for patients with indolent lymphoma (FORT): a randomised phase 3 non-inferiority trial. Lancet Oncol 2014;15:457-63. [Crossref] [PubMed]
- Janikova A, Bortlicek Z, Campr V, et al. Radiotherapy with rituximab may be better than radiotherapy alone in first-line treatment of early-stage follicular lymphoma: is it time to change the standard strategy? Leuk Lymphoma 2015;56:2350-6. [Crossref] [PubMed]
- Ruella M, Filippi AR, Bruna R, et al. Addition of Rituximab to Involved-Field Radiation Therapy Prolongs Progression-free Survival in Stage I-II Follicular Lymphoma: Results of a Multicenter Study. Int J Radiat Oncol Biol Phys 2016;94:783-91. [Crossref] [PubMed]
- Cencini E, Puccini B, Rigacci L, et al. Radiotherapy plus rituximab as first-line regimen for localized follicular lymphoma. Leuk Lymphoma 2018;59:1420-6. [Crossref] [PubMed]
- Witzens-Harig M, Hensel M, Unterhalt M, et al. Treatment of limited stage follicular lymphoma with Rituximab immunotherapy and involved field radiotherapy in a prospective multicenter Phase II trial-MIR trial. BMC Cancer 2011;11:87. [Crossref] [PubMed]
- Herfarth K, Borchmann P, Schnaidt S, et al. Rituximab With Involved Field Irradiation for Early-stage Nodal Follicular Lymphoma: Results of the MIR Study. HemaSphere 2018;2:e160 [Crossref] [PubMed]
- MacManus M, Fisher R, Roos D, et al. Randomized Trial of Systemic Therapy After Involved-Field Radiotherapy in Patients With Early-Stage Follicular Lymphoma: TROG 99.03. J Clin Oncol 2018;36:2918-25. [Crossref] [PubMed]
- Nastoupil LJ, Sinha R, Byrtek M, et al. Outcomes following watchful waiting for stage II-IV follicular lymphoma patients in the modern era. Br J Haematol 2016;172:724-34. [Crossref] [PubMed]
- Ardeshna KM, Qian W, Smith P, et al. Rituximab versus a watch-and-wait approach in patients with advanced-stage, asymptomatic, non-bulky follicular lymphoma: an open-label randomised phase 3 trial. Lancet Oncol 2014;15:424-35. [Crossref] [PubMed]
- Solal-Céligny P, Bellei M, Marcheselli L, et al. Watchful waiting in low-tumor burden follicular lymphoma in the rituximab era: results of an F2-study database. J Clin Oncol 2012;30:3848-53. [Crossref] [PubMed]
- Kahl BS, Hong F, Williams ME, et al. Rituximab extended schedule or re-treatment trial for low-tumor burden follicular lymphoma: eastern cooperative oncology group protocol e4402. J Clin Oncol 2014;32:3096-102. [Crossref] [PubMed]
- Al-Tourah AJ, Gill KK, Chhanabhai M, et al. Population-based analysis of incidence and outcome of transformed non-Hodgkin’s lymphoma. J Clin Oncol 2008;26:5165-9. [Crossref] [PubMed]
- Trotman J, Barrington SF, Belada D, et al. Prognostic value of end-of-induction PET response after first-line immunochemotherapy for follicular lymphoma (GALLIUM): secondary analysis of a randomised, phase 3 trial. Lancet Oncol 2018;19:1530-42. [Crossref] [PubMed]
- Meignan M, Cottereau AS, Versari A, et al. Baseline Metabolic Tumor Volume Predicts Outcome in High-Tumor-Burden Follicular Lymphoma: A Pooled Analysis of Three Multicenter Studies. J Clin Oncol 2016;34:3618-26. [Crossref] [PubMed]
- Cottereau AS, Versari A, Luminari S, et al. Prognostic model for high-tumor-burden follicular lymphoma integrating baseline and end-induction PET: a LYSA/FIL study. Blood 2018;131:2449-53. [Crossref] [PubMed]
- Strati P, Ahmed MA, Nastoupil LJ, et al. Pretreatment SUVmax may influence the clinical benefit of BR over R-CHOP in patients with previously untreated FL. Leuk Lymphoma 2020;61:1380-7. [Crossref] [PubMed]
- Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone R Blood 2005;106:3725-32. [Crossref] [PubMed]
- Marcus R, Imrie K, Belch A, et al. CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood 2005;105:1417-23. [Crossref] [PubMed]
- Herold M, Haas A, Srock S, et al. Rituximab added to first-line mitoxantrone, chlorambucil, and prednisolone chemotherapy followed by interferon maintenance prolongs survival in patients with advanced follicular lymphoma: an East German Study Group Hematology and Oncology Study. J Clin Oncol 2007;25:1986-92. [Crossref] [PubMed]
- Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 2013;381:1203-10. [Crossref] [PubMed]
- Federico M, Luminari S, Dondi A, et al. R-CVP versus R-CHOP versus R-FM for the initial treatment of patients with advanced-stage follicular lymphoma: results of the FOLL05 trial conducted by the Fondazione Italiana Linfomi. J Clin Oncol 2013;31:1506-13. Erratum in: J Clin Oncol 2014;32:1095. [Crossref] [PubMed]
- Flinn IW, van der Jagt R, Kahl BS, et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood 2014;123:2944-52. [Crossref] [PubMed]
- Luminari S, Ferrari A, Manni M, et al. Long-Term Results of the FOLL05 Trial Comparing R-CVP Versus R-CHOP Versus R-FM for the Initial Treatment of Patients With Advanced-Stage Symptomatic Follicular Lymphoma. J Clin Oncol 2018;36:689-96. [Crossref] [PubMed]
- Flinn IW, van der Jagt R, Kahl B, et al. First-Line Treatment of Patients With Indolent Non-Hodgkin Lymphoma or Mantle-Cell Lymphoma With Bendamustine Plus Rituximab Versus R-CHOP or R-CVP: Results of the BRIGHT 5-Year Follow-Up Study. J Clin Oncol 2019;37:984-91. [Crossref] [PubMed]
- Hiddemann W, Barbui AM, Canales MA, et al. Immunochemotherapy With Obinutuzumab or Rituximab for Previously Untreated Follicular Lymphoma in the GALLIUM Study: Influence of Chemotherapy on Efficacy and Safety. J Clin Oncol 2018;36:2395-404. [Crossref] [PubMed]
- Marcus R, Davies A, Ando K, et al. Obinutuzumab for the First-Line Treatment of Follicular Lymphoma. N Engl J Med 2017;377:1331-44. [Crossref] [PubMed]
- van Oers MHJ, Van Glabbeke M, Giurgea L, et al. Rituximab maintenance treatment of relapsed/resistant follicular non-Hodgkin’s lymphoma: long-term outcome of the EORTC 20981 phase III randomized intergroup study. J Clin Oncol 2010;28:2853-8. [Crossref] [PubMed]
- Morschhauser F, Fowler NH, Feugier P, et al. Rituximab plus Lenalidomide in Advanced Untreated Follicular Lymphoma. N Engl J Med 2018;379:934-47. [Crossref] [PubMed]
- Bachy E, Seymour JF, Feugier P, et al. Sustained Progression-Free Survival Benefit of Rituximab Maintenance in Patients With Follicular Lymphoma: Long-Term Results of the PRIMA Study. J Clin Oncol 2019;37:2815-24. [Crossref] [PubMed]
- Salles GA, Seymour JF, Feugier P, et al. Updated 6 Year Follow-Up Of The PRIMA Study Confirms The Benefit Of 2-Year Rituximab Maintenance In Follicular Lymphoma Patients Responding To Frontline Immunochemotherapy. Blood 2013;122:509. [Crossref]
- Salles G, Seymour JF, Offner F, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet 2011;377:42-51. [Crossref] [PubMed]
- Hill BT, Nastoupil L, Winter AM, et al. Maintenance rituximab or observation after frontline treatment with bendamustine-rituximab for follicular lymphoma. Br J Haematol 2019;184:524-35. [Crossref] [PubMed]
- Madsen C, Clausen MR, Plesner TL, et al. Up-front rituximab maintenance improves outcome in patients with follicular lymphoma: a collaborative Nordic study. Blood Adv 2018;2:1562-71. [Crossref] [PubMed]
- Bech RS, Nielsen KL, Larsen TS, et al. Real world data on rituximab maintenance therapy after frontline immunochemotherapy in grade 1-3a follicular lymphoma. Br J Haematol 2018;182:297-301. [Crossref] [PubMed]
- Owen C, Stewart DA. Obinutuzumab for the treatment of lymphoproliferative disorders. Expert Opin Biol Ther 2012;12:343-51. [Crossref] [PubMed]
- Mössner E, Brünker P, Moser S, et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood 2010;115:4393-402. [Crossref] [PubMed]
- Patz M, Isaeva P, Forcob N, et al. Comparison of the in vitro effects of the anti-CD20 antibodies rituximab and GA101 on chronic lymphocytic leukaemia cells. Br J Haematol 2011;152:295-306. [Crossref] [PubMed]
- Dalle S, Reslan L, Besseyre de Horts T, et al. Preclinical studies on the mechanism of action and the anti-lymphoma activity of the novel anti-CD20 antibody GA101. Mol Cancer Ther 2011;10:178-85. [Crossref] [PubMed]
- Alduaij W, Ivanov A, Honeychurch J, et al. Novel type II anti-CD20 monoclonal antibody (GA101) evokes homotypic adhesion and actin-dependent, lysosome-mediated cell death in B-cell malignancies. Blood 2011;117:4519-29. [Crossref] [PubMed]
- Herter S, Herting F, Mundigl O, et al. Preclinical activity of the type II CD20 antibody GA101 (obinutuzumab) compared with rituximab and ofatumumab in vitro and in xenograft models. Mol Cancer Ther 2013;12:2031-42. [Crossref] [PubMed]
- Ghielmini M, Schmitz S-FH, Cogliatti SB, et al. Prolonged treatment with rituximab in patients with follicular lymphoma significantly increases event-free survival and response duration compared with the standard weekly x 4 schedule. Blood 2004;103:4416-23. [Crossref] [PubMed]
- Martinelli G, Schmitz S-FH, Utiger U, et al. Long-term follow-up of patients with follicular lymphoma receiving single-agent rituximab at two different schedules in trial SAKK 35/98. J Clin Oncol 2010;28:4480-4. [Crossref] [PubMed]
- Taverna CJ, Bassi S, Hitz F, et al. Rituximab Maintenance Treatment for a Maximum of 5 Years In Follicular Lymphoma: Safety Analysis of the Randomized Phase III Trial SAKK 35/03. Blood 2010;116:1802. [Crossref]
- Taverna C, Martinelli G, Hitz F, et al. Rituximab Maintenance for a Maximum of 5 Years After Single-Agent Rituximab Induction in Follicular Lymphoma: Results of the Randomized Controlled Phase III Trial SAKK 35/03. J Clin Oncol 2016;34:495-500. [Crossref] [PubMed]
- Lockmer S, Østenstad B, Hagberg H, et al. Chemotherapy-Free Initial Treatment of Advanced Indolent Lymphoma Has Durable Effect With Low Toxicity: Results From Two Nordic Lymphoma Group Trials With More Than 10 Years of Follow-Up. J Clin Oncol 2018;JCO1800262 [Crossref] [PubMed]
- Fowler NH, Davis RE, Rawal S, et al. Safety and activity of lenalidomide and rituximab in untreated indolent lymphoma: an open-label, phase 2 trial. Lancet Oncol 2014;15:1311-8. [Crossref] [PubMed]
- Martin P, Jung S-H, Pitcher B, et al. A phase II trial of lenalidomide plus rituximab in previously untreated follicular non-Hodgkin’s lymphoma (NHL): CALGB 50803 (Alliance). Ann Oncol 2017;28:2806-12. [Crossref] [PubMed]
- Kimby E, Rondeau S, Vanazzi A, et al. Rituximab Plus Lenalidomide Versus Rituximab Monotherapy in Untreated Follicular Lymphoma Patients in Need of Therapy. First Analysis of Survival Endpoints of the Randomized Phase-2 Trial SAKK 35/10. Blood 2016;128:1099. [Crossref]
- Zucca E, Rondeau S, Vanazzi A, et al. Short regimen of rituximab plus lenalidomide in follicular lymphoma patients in need of first-line therapy. Blood 2019;134:353-62. [Crossref] [PubMed]
- Delfau-Larue MH, Boulland ML, Beldi-Ferchiou A, et al. Lenalidomide/rituximab induces high molecular response in untreated follicular lymphoma: LYSA ancillary RELEVANCE study. Blood Adv 2020;4:3217-23. [Crossref] [PubMed]
- Nastoupil LJ, Westin JR, Hagemeister FB, et al. Results of a Phase II Study of Obinutuzumab in Combination with Lenalidomide in Previously Untreated, High Tumor Burden Follicular Lymphoma (FL). Blood 2019;134:125. [Crossref]
Cite this article as: Luminari S, Tarantino V. Frontline management for follicular lymphoma patients: a narrative review. Ann Lymphoma 2021;5:10.