
Minimal residual disease in follicular lymphoma
Introduction
Although generally incurable in advanced stage, outcomes of follicular lymphoma (FL) have improved continuously over the last two decades. Thanks to novel treatment modalities including the anti-CD20 antibodies rituximab and obinutuzumab, myeloablative chemo- or radio-chemotherapy followed by autologous stem cell transplantation (ASCT) and the availability of new biologic agents, a significant improvement of response rates, response duration and overall survival (OS) has been achieved with a substantial impact on long term outcome. Median OS in advanced stage FL is now approaching nearly 20 years, with this approaching the life-expectancy of the general population matched by gender and age (1,2).
Despite the overall improvement in first line treatment, progression-free survival (PFS) and OS of patients with clinical remission at the end of induction (EOI) and the end of treatment (EOT) varies substantially among individual patients. The life expectancy of FL-patients is mainly determined by the response to initial treatment and the time point of relapse (3). Thus, treatment recommendations during the course of the disease are challenging and range from a watch-and-wait strategy to single-agent treatment with an anti-CD20 monoclonal antibody, or different immuno-chemotherapy combinations up to intensive multimodal concepts including ASCT (2,4). Despite long-term disease control in the majority of patients, approximately 20% of patients progress within 2 years after immunochemotherapy with a short 5-year OS of only 50% (3,5).
A number of baseline clinical, histological and biological predictors have been identified in FL like the “Follicular Lymphoma International Prognostic Indexes” FLIPI (6), FLIPI-1 and FLIPI-2 based on clinical parameters, and m7-FLIPI (7) including genetic risk factors to define prognostic subgroups. With the exception of the “late” prognostic factor progression of disease within 2 years (POD24) these take into account only pretherapeutic risk factors. Although prognostic, they are not used for risk adapted treatment strategies.
As in general FL remains an incurable disease, the duration of remission in individual patients is determined by the quantitative extent of residual lymphoma cells after treatment [minimal residual disease (MRD)] as well as their proliferation kinetics during follow-up. Both parameters reflect the individual disease biology that determines long-term prognosis. Thus, the concept of management of indolent lymphomas relies on the most efficient reduction of the tumor burden as relevant step to achieve a long-term disease control. In recent years the concept of MRD detection has gained relevant interest as a post-treatment outcome predictor in lymphoma and a considerable amount of data has been generated in the field (8-11).
In combination with imaging tools such as computed tomography (CT) or 18F-fluorodeoxyglucose positron emission tomography (18F-FDG-PET) CT, laboratory based MRD tools could allow a more accurate assessment of remission and thereby the identification of different risk groups after treatment, an important step towards a risk adapted treatment approach (12). Possible scenarios for risk adapted treatment strategies are a stop or change of treatment, the direction of maintenance or initiation of preemptive therapy, treatment intensification or consolidation (9).
Several methods have been proven to be useful for MRD monitoring in the context of indolent lymphomas. Different techniques for MRD detection vary not only in a different performance in terms of applicability, accuracy, sensitivity and specificity but also with respect to disease specifications and the availability of diagnostic material with sufficient tumor cells to identify an MRD marker (13-15).
This review discusses major technical aspects of the available methods for the analysis of MRD in FL with their individual advantages and disadvantages and the significant clinical messages that have been derived from the application of MRD monitoring in controlled clinical trials during the last decade.
Methods for MRD analyses
Different methods are available to detect residual lymphoma cells in peripheral blood (PB) and bone marrow (BM) during and after treatment below the sensitivity threshold of common diagnostic techniques. A general requirement of MRD techniques is that a minimum sensitivity of 10E-4 to 10E-5 should be reached to accurately define the MRD-based risk groups. Currently available MRD methods vary according to sensitivity, specificity and accuracy of target quantification and have individual technical biases (13,14,16-18). Overall, MRD techniques have improved over the last 10 years especially in their quality and the practical knowledge of their use (13,14,19). New high-throughput PCR sequencing techniques [next-generation sequencing (NGS)] for MRD detection have been developed with the option to reach a higher sensitivity and specificity, but its application still needs validation in clinical studies as well as standardization for a broad application in clinical trials. Each method has specific advantages and disadvantages that will be described (Table 1) and critically discussed in the following paragraphs.
Table 1
| qPCR | ddPCR | IG-NGS | |
|---|---|---|---|
| Advantages | Fully standardized | No need of standard curve quantification | Patient independent approach |
| Regular Euro-MRD QC rounds | Regular Euro-MRD QC rounds | High specificity | |
| Short turnaround time (when marker defined) and cost-effective | Short turnaround time (when marker defined) and cost-effective | Sensitivity 10E-6 | |
| Prognostically relevant time points defined | Direct quantification | Direct quantification | |
| Guidelines and recommendations for reporting | Guidelines and recommendations for reporting | High-throughput method | |
| Disadvantages | Sensitivity limited to 10E-5 | Sensitivity limited to 10E-5 | Expensive |
| Marker screening and sequencing, ASO primer design required | Marker screening and sequencing, ASO primer design required | Long turnaround time | |
| Patient specific approach | Patient specific approach | Validation in clinical trials lacking | |
| Grey zone of non-quantifiable results | Grey zone of non-quantifiable results (less than qPCR) | Not standardized, no guidelines or QC | |
| Requires high infiltrated diagnostic sample to establish a serial dilution | Low-throughput method | Laborious (high bioinformatic input is needed) | |
| Low-throughput method | Risk of contamination |
MRD, minimal residual disease; qPCR, quantitative real-time PCR; ddPCR, digital droplet PCR; IG, immunoglobulin; NGS, next-generation sequencing; QC, quality control; ASO, allele-specific oligonucleotide.
Flow-cytometry (FC) based methods
FC is frequently used in routine diagnostics of hematological diseases. FC is broadly available and one major advantage is to get a quick result in a few hours compared to more laborious molecular methods. The principle of FC is the detection of the immunoglobulin (IG) light-chain restriction in B-cell lymphomas and the identification of immunophenotypic abnormalities. However, while FC is an established method for MRD detection in chronic lymphocytic leukemia (CLL) (20-22) and mantle cell lymphoma (MCL) (23), there are no established FC panels for MRD detection in FL. While the EuroFlow consortium offered a standardized approach for effective FL diagnostics (24), no standardized approach for FC based MRD detection has been implemented for an application in prospective clinical trials. This can be explained in part by the fact that CD10 as a hallmark for histopathology in FL is frequently down regulated when FL becomes leukemic (25).
PCR based methods
Quantitative real-time PCR (qPCR)
qPCR is the most frequently used and best validated method for MRD detection in FL. Uniform guidelines and quality control (QC) rounds are in place and the majority of MRD data in FL have been produced by using qPCR.
The sensitivity of qPCR routinely reaches from 10E-4 to 10E-5 (1 lymphoma cell in 10,000 to 100,000 non-involved cells) in an adequate DNA amount. PCR data in FL are mainly based on MRD assessment of t(14;18) positive cells, the genetic hallmark of FL (26). The t(14;18) translocation results from the juxtaposition between chromosome 18 and chromosome 14 including the immunoglobulin heavy-chain (IGH) genes and the BCL-2 gene (26,27) and is a robust and stable target for MRD analysis. It is however, restricted only to those 60% of FL cases with a PCR detectable t(14;18) breakpoint. About 40% of chromosome 18 breakpoints occur in the major breakpoint region (MBR) of the BCL-2 gene spanning a region of 150 bp. There are two other breakpoint regions that have to be taken into account for an efficient PCR strategy, the 3'MBR subcluster located 4 kb downstream of the MBR encompassing a region of 3.8 kb [9% of all t(14;18) breakpoints] and the minor cluster region (MCR) located 20 kb 3' of the MBR covering a region of about 500 bp [10–15% of all t(14;18) breakpoints]. A number of variant translocations have been described that cannot be routinely assessed by PCR (28,29). The translocation partner of chromosome 18 is the joining region of the IGH gene on chromosome 14, where the breakpoints are almost exclusively located within one of the six germline JH regions encompassing allowing PCR amplification of the translocation (26).
The t(14;18) qPCR assays employ a forward primer and a fluorogenically labelled hybridization probe positioned on chromosome 18, the reverse primer is located in the consensus germline JH gene segment of chromosome 14 (Figure 1). The advantage of this approach is its uniform applicability to t(14;18) MBR positive FL patients without the need of sequencing of the junctional region. One disadvantage is the considerable variability of the PCR product size, that can vary from 150 up to 500 bp depending on breakpoint location and the N-region length of the individual t(14;18) translocation. This has to be considered when comparing MRD results among individuals. The EuroMRD consortium, founded in 2001 as a division of ESLHO (European Scientific foundation for Laboratory Hemato Oncology EuroMRD network), has standardized qPCR approaches for the translocation and moreover has designed plasmid standards also for the assessment of the variant rare breakpoints. These plasmids are available on request (http://www.euromrd.org).
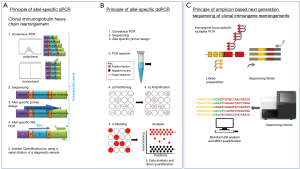
The restriction of t(14;18) qPCR to those FL with a PCR detectable translocation lead to the exploration of clonal IG light-chain and IGH rearrangements as PCR targets. A clonal IGH rearrangement is detectable in more than 80–95% of B-cell neoplasia (30) and can be successfully used for MRD assessment. Sequencing of the junctional region of rearranged IGH genes allows the identification of the tumor specific VH-DH-JH rearrangement and by this an allele-specific oligonucleotide (ASO) primer can be designed for a sensitive qPCR approach (31,32). Because each patient’s clonal IG rearrangement is unique, the assay characteristic (sensitivity and the quantitative range) differ among patients and therefore need to be individually established.
One challenge of using clonal IGH rearrangements as MRD targets is the fact that mature B-cell lymphomas, specifically FL, feature somatic hypermutation (SHM) of the IGH target region as well as ongoing SHM during clonal evolution. This can hamper accurate primer binding in the IGH region and might underestimate the true MRD-value. Approaches with IGH probes placed in the consensus (framework) regions of the respective IGH gene try to circumvent this problem (31) and have been successfully applied also in somatically mutated subtypes (31,33).
One further issue for IGH based MRD detection in FL is the non-universal presence of a substantial tumor infiltration in the diagnostic PB or BM sample. This is a prerequisite for target identification and for the establishment of a reference standard curve for quantitation of follow-up samples by qPCR. For generating a reference standard curve the lymphoma cell infiltration should ideally be ≥5% of FL cells. As this is sometimes difficult to assess, cloning of rearrangements and the usage of plasmids for MRD quantification may be an alternative (34). This approach has been successfully used for MRD detection in a large prospective phase III trial in the first-line treatment of FL (10), but it is a laborious process that requires individually cloned plasmids and the sensitivity and the quantitative range of qPCR has to be assessed separately for each individual qPCR assay. Therefore, new technologies like high-throughput sequencing might overcome this limitation in the future (13).
For the application of MRD in clinical studies, uniform guidelines for application, sensitivity and quantitative range as well as data interpretation are a substantial requirement (35). This has been a major task of the EuroMRD consortium, founded in 2001 as a division of ESLHO (European Scientific foundation for Laboratory Hemato Oncology, see www.EuroMRD.org). Within the EuroMRD consortium a working group focusing on the specific requirements of MRD in lymphomas has advanced standardization and data interpretation in this setting and also offers regular control rounds to monitor the performance of the participating laboratories and to further improve and standardize qPCR analyses.
Digital PCR
One of the major limitations of qPCR is the detection of MRD below the quantitative range [positive non quantifiable (PNQ)]. After effective immunochemotherapy, 2/3 of patients achieve PCR positivity within this grey-zone. The occurrence of PNQ may be due to technical reasons and can reflect true low-level residual disease, or non-specific target amplification, resulting in a false positive result (14,36).
Digital droplet PCR (ddPCR), is a PCR based method for absolute quantification of MRD via target compartmentalization in a water-oil emulsion with patient-specific primers and/or -probes. ddPCR is less sensitive to PCR inhibitors based on endpoint amplification (14) and reaches a sensitivity comparable to qPCR (37-39). ddPCR is able to provide an accurate quantitative MRD result in 20–45% of positive non-quantifiable samples quantified by qPCR thanks to its absolute quantification. This is advantageous for MRD diagnostics in a low-throughput setting and allows MRD quantification also in those patients in which a standard curve cannot be reliably generated due to low tumor burden at diagnosis. However, the laborious design of patient-specific assays cannot be avoided.
Guidelines for ddPCR-based MRD detection have been defined for the application in lymphomas by the lymphoma working group of the EuroMRD consortium (36). In a recently published series of patients with MCL, ddPCR was superior to qPCR since it provided more robust quantification at positivity in the range between 10E-4 and 10E-5 (36). This is mainly observed for qPCR with ASO primers while for the translocation t(14;18) both methods yield comparable MRD results (14).
The predictive value of ddPCR in comparison to qPCR has been confirmed in FL and MCL (14,37). In a comparative analysis of qPCR and NGS the prognostic relevance of ddPCR could be demonstrated in prospective trials of the European MCL Network. The results have been submitted to the International Conference on Malignant Lymphoma 2021 (40).
NGS
NGS is a high-throughput sequencing method that can also be applied for MRD detection in hematological diseases by addressing the clonal IG or T-cell receptor (TR) gene rearrangements characterizing the disease. NGS may overcome some limitations of PCR-based methods and has the potential of a higher sensitivity and specificity. NGS based MRD assessment does not require the laborious design of patient-specific tests as the same multiplex primer set is used for marker screening and MRD assessment.
As in PCR based methods, the IG rearrangement representing the clonal fingerprint of the disease is determined by marker screening and subsequently traced for MRD assessment. For marker identification by amplicon NGS, the initial step is a multiplex PCR for the amplification of the clonal IG or TR gene rearrangement, followed by a second-round PCR with barcoded primers for library preparation and subsequent high-throughput sequencing. Also a one-step PCR with barcoded multiplex primers can be used.
Marker identification by NGS can be challenging and requires a substantial infiltration of PB or BM by lymphoma cells, as unrelated B- or T-cell clones can contribute to a significant background of IG and TR sequences not related to the malignant clone (41). In leukemic diseases like acute lymphocytic leukemia (ALL), MCL and CLL, a 5% frequency cut-off for the most abundant clonotype can be used to allocate a clone as disease related clonotype (13,18,19,42,43).
In our hands, a correction of marker reads according to the number of cell equivalents sequenced in the reaction resulted in a more precise identification of the lymphoma specific clonotype when a threshold for the most abundant clonotype of ≥1% in a polyclonal background is chosen (18,44).
Notably, this requires the usage of reference standard DNA that is spiked into each sample in known copy number to correct for amplification biases and to allow NGS-MRD quantification by normalization of NGS reads to cell copy numbers (45).
Our group has shown that marker screening by IG-NGS in unselected diagnostic PB and BM of patients receiving first-line treatment for advanced FL was successful in only 50% of the cases independent of the use of PB or BM (44), the latter expected to have a higher degree of infiltration. This detection rate is lower than that reached with PCR with consensus primers and gene scan analysis (10) and underlines the importance of infiltrated tumor tissue for IG-NGS MRD marker screening. When IGK light chain clonotypes were additionally sequenced, lymphoma related IG clonotype could be detected in 64% of diagnostic samples (44). Therefore, analysis of formalin-fixed diagnostic lymph-node tissue might be necessary for screening and identification of the tumor-specific clonotypic at least in typical nodal types of lymphomas.
A further issue in amplicon-based sequencing strategies are somatic mutations in primer binding sites hampering proper primer binding. This occurs mainly in the IGH region during B-cell maturation and is particularly important in mature B-cell malignancies like FLs, diffuse large B-cell lymphomas and multiple myeloma (46-48), where the clonal IG index sequence might harbor considerable rates of SHM resulting in a decreased amplification efficiency.
Alternatively, incomplete IGHD-IGHJ rearrangements and IGK gene rearrangements could be used as targets for MRD (49). Both rearrangement types are mainly unmutated. Incomplete rearrangements in the IGH locus do not contain SHMs in the majority of cases because transcription starting from the promoters in the V gene segments does not occur. The finding of hypermutation in a small proportion of incomplete DH-JH rearrangements suggests important biological implications concerning the process of SHM. The rearrangements of the IGK genes can also be an important complementary MRD target, as in rearrangements involving the kappa deletion element (Kde) no SHM can occur after Kde recombination, since the deletion of the JK-CK introns removes the IGK enhancer essential for SHM (50).
MRD assessment by IG-NGS
For MRD analysis by NGS, the clonally rearranged IG loci are sequenced by using the same consensus primer set in follow-up samples, followed by sequencing library construction and data evaluation. This allows sensitive detection of lymphoma-specific clonotypes with a sensitivity of up to 10E-6, dependant from the amount of input DNA.
Most published data in the literature using IG-NGS for MRD assessment refer to a commercially available assay using the ClonoSeq platform (Adaptive, Seattle, WA, USA) which has been tested in a broad array of hematological malignancies like CLL (51) and ALL (43,52) and B-cell lymphomas (13). A comparative analysis of our group in patients with ALL, MCL and FLs addressing the potential of NGS to overcome some of the limitations of ASO-qPCR have shown that both methods have comparable sensitivity, and NGS has the potential for further increased sensitivity and specificity (13). Recent studies have shown that undetectable MRD determined by IG-NGS below the level of 10E-5 reveals advantageous prognostic subgroups in patients with CLL (53) and multiple myeloma (54), so it would be very attractive to investigate the potential of IG-NGS also in FL.
Like in PCR-based MRD methods, the sensitivity of MRD detection by NGS is depending on the number of analyzed cells corresponding to the amount of DNA. A sequencing depth of 10E-6 requires sufficient DNA of at least one million cells, equivalent to 10–12 µg DNA, assuming high quality DNA without PCR inhibitors. This could be critical to achieve, in particular after a B-cell depleting therapy or during maintenance treatment.
The Euro Clonality NGS working group of ESLHO has put great efforts in the development of new IG/TR NGS assays as well as their standardization and validation (19,45,55) to broaden access to NGS based techniques for clonality assessment, MRD detection and IG/TR repertoire analyses. This network approach includes the definition of guidelines for application, required sensitivity and data interpretation.
Correct MRD quantification by amplicon NGS requires the use of standardized internal controls to correct for amplification biases and to allow NGS-MRD quantification by normalization of NGS reads to cell copy numbers. This is of particular importance in the situation of low numbers of polyclonal background B-cells, as MRD quantification by counting number of index sequences and dividing them by the total number of sequenced amplicons might lead to a considerable overestimation of MRD.
Correct and reproducible IG-NGS MRD quantification is complex and requires a bioinformatic platform for the standardization of input processing, selection and filtering of data, comparative calculation and visualization of immunogenetic annotation of sequences. The published pipeline ARResT/Interrogate of the EuroClonality-NGS consortium is already being used for standardized MRD in NGS (45,56) and has recently been adapted for MRD quantification in FL, where excessive clonal heterogeneity requires systematic merging of IG clones for MRD calculation.
In the comparative analysis of qPCR, ddPCR and NGS in study patients with MCL, the prognostic relevance of all three methods could be demonstrated (40). Our comparative data of IG-NGS with qPCR in FL show adequate sensitivity and reproducibility in (44) with the EuroClonality-NGS approach, but the application is limited to those patients with successful IG-NGS marker screening in diagnostic PB and BM. Therefore, analysis of formalin-fixed diagnostic lymph-node tissue might be additionally used for marker screening in nodal lymphomas.
Sampling and prognostic relevant time points for MRD detection
For MRD assessment, circulating lymphoma cells or cell free DNA (cfDNA) can be analyzed. Data in FL mainly rely on the assessment of circulating lymphoma cells in PB and BM, the latter being appraised as the most reliable specimen for MRD analysis due to the rapid clearance of FL from PB by anti-CD20 monoclonal antibody rituximab treatment (57). Therefore, most studies investigating the prognostic impact of MRD after intensive treatment including ASCT used BM for MRD assessment (11,58-61). In both specimen it could be shown that MRD positivity is correlated with a subsequent clinical relapse and a worse PFS and/or OS (8,62-70).
Due to the easier access PB has gained importance and the prognostic impact of MRD even under anti-CD20 monoclonal antibody treatment has been proven in the phase 3 Gallium trial (10). This prospective analysis gave important hints on the timing of MRD analysis and showed for the first time that the prognostic impact of the MRD status in PB at interim staging was a strong prognostic factor.
MRD responses were achieved early (94% in PB in the obinutuzumab arm) and with a median follow-up of 57 months, MRD response at interim staging shows a 64% reduction in the risk of a PFS event relative to MRD-positive status (HR, 0.36; 95% CI: 0.25–0.53; P<0.0001). Moreover, PFS was longer for “early responders” in comparison to “late responders” (those being MRD-positive at the mid of induction, but MRD-negative at EOT) and to patients not achieving MRD response (4-year PFS, 80% for the “early responders” vs. 60% for the “late responders” vs. 30% for “always MRD positive patients. In the setting of highly effective treatment protocols, BM probably is the more significant sample for MRD assessment especially as it is known to be cleared from lymphoma cells later and less effective than PB (10). This is in particular important when a therapeutic intervention is considered according to the MRD result. Published evidence for PB or BM at different investigational time points is summarized in Table 2.
Table 2
| Time point | Prognostic significance | Material |
|---|---|---|
| Diagnosis | Rambaldi, 2005 (61) | PB, BM |
| Pott, 2006 (66) | PB, BM | |
| Galimberti, 2014 (71) | BM | |
| Zohren, 2015 (65) | PB | |
| Interim staging | Hirt, 2008 (67) | PB |
| Pott, 2018 (10) | PB | |
| EOT | Ladetto, 2008 (60) | BM |
| Ladetto, 2013 (11) | PB, BM | |
| Rambaldi, 2005 (61) | BM | |
| Pott, 2018 (10) | PB, BM | |
| Post remission evaluation | ||
| 3–12 months after induction | Ladetto, 2008 (60) | BM |
| Ladetto, 2013 (11) | BM | |
| Pott, 2018 (10) | PB | |
| López-Guillermo, 1998 (69) | PB, BM | |
| 12–24 months after induction | Pott, 2018 (10) | PB |
| Galimberti, 2014 (71) | BM | |
| López-Guillermo, 1998 (69) | PB, BM | |
MRD, minimal residual disease; EOT, end of treatment; PB, peripheral blood; BM, bone marrow.
Clinical significance of FDG-PET
18F-FDG-PET/computed tomography is a highly sensitive method for baseline staging and response assessment after therapy in all histological subtypes of FDG avid lymphomas. It has also been shown, that PET response is an independent outcome predictor in several lymphoma subtypes including FL (72,73).
The prognostic role of PET measuring total metabolic tumor volume (TMTV) and metabolic response has been shown in a pooled analysis of 159 FL patients from 3 prospective trials (LYSA, FIL) evaluating imaging biomarkers for early risk stratification. TMTV and PET at EOI correlated significantly with the 5-year PFS (74). The strong prognostic significance of PET at EOI was also confirmed in the randomized phase 3 GALLIUM trial, where PET response after first-line immunochemotherapy identified different risk groups. The 77% of patients who achieved a metabolic response had a superior PFS and OS, independent of treatment arm (72). These studies confirmed that 18F-FDG-PET has the highest sensitivity and accuracy of all imaging techniques and is the preferred technique for initial staging and response assessment. Interestingly, a high pretherapeutic TMTV seems to correlate with circulating tumor cells and the abundancy of cfDNA in untreated FL at diagnosis and is associated with adverse outcome after first-line treatment (75). This association shows a relation between the tissue tumor burden and circulating tumor burden, measuring these parameters and integrating them in prognostic models might provide even more precise information for tailored treatment approaches in the future.
Given the high sensitivity of both, PET and MRD, it seems reasonable to combine both methods for response assessment. This concept was tested for the first time in a subset of 41 patients of the Italian phase III (FOLL05) FL trial in which combined data on MRD and PET at EOT where available (76). With the limitation of a small series of patients the results indicated that the combination of the two approaches improve the ability to identify subgroups of high and low risk patients in FL, and therefore can be regarded as complementary response assessment tools. In 298 patients of the Gallium trial with both parameters analysed at EOI, the patient group with complete metabolic response (CMR) and MRD-negative response (n=246) had a favorable prognosis, the 2.5-year PFS (from EOI) was 85% (95% CI: 80–89%), compared to 69% (95% CI: 40–86%) in the PET and MRD-positive group (12).
These results confirm that PET and MRD at the EOI treatment are complementary techniques for prediction of outcome, suggesting that the circulating and tissue tumor burden at diagnosis is not equivalent with regard to therapeutic efficacy of a certain treatment.
Significant clinical messages for the use of MRD in FL
Large multicenter studies with FL patients analyzing the MRD detection were done to prove the value of MRD during and after treatment in terms of early identification of a molecular relapse.
A main conclusion from these clinical trials is that the combination of imaging and MRD tools for response assessment allows a more precise characterization of individual lymphoma biology.
In the last decade the accumulated knowledge has shown that MRD is a reliable tool for highly sensitive response assessment and identification of patients with an adverse prognosis.
Relevant experiences for clinical treatment are listed in the following:
- Staging of FL at diagnosis is mainly based on imaging techniques using computed tomography scan. In early stage FL, circulating lymphoma cells at diagnosis can be detected in more than 2/3 of FL with localized disease by imaging (37,77). This identifies a more extensive tumor burden than imaging techniques do, this is supported by the fact that patients with disseminated disease have a poorer outcome. In early stage FL, also the MRD status during follow-up after curative intended radiotherapy with or without rituximab has prognostic implications (77).
- In advanced disease, achievement of MRD response by conventional chemotherapy was only possible in a small number of FL patients (57). The goal to reach MRD response was possible in the pre-rituximab era by treatment with intensive chemotherapy followed by ASCT in over 70% of the FL patients (58,59).
- MRD negativity in 50–80% of patients can be reached by the integration of Rituximab in combination with chemotherapy (11,14,62,71). This new immunotherapeutic approach has pioneered the systematic MRD assessment in FL and established MRD as post-treatment parameter for prognosis.
- Data from randomized trials started in the 2000er years showed that independent from the treatment modality (ASCT with and without Rituximab or conventional immunochemotherapy alone) MRD response affected outcome and was an independent prognostic factor. An overview about the results is summarized in Table 3.
Table 3
Relevant literature demonstrating the prognostic relevance of MRD responseTrial Authors Regimen N Survival MRD pos. vs. neg. Time point Method Phase MRD P value MRD results from clinical trials at first line treatment East German Study Group Hematology and Oncology Trial 39 (OSHO 39), ID 00269113 Hirt et al., Br J Haematol, 2008 (67) R-MCP vs. MCP 43 2-year OS 95% vs. 82% Post induction qPCR III <0.03 GITMO trial, NCT00435955 Ladetto et al., Blood, 2008 (60) R-CHOP vs. R-HDS (ASCT) 73 4-year OS, 4-year PFS 80% vs. 81%, 31% vs. 68% EOI nPCR III 0.96, <0.001 First line indolent trial (FIT), NCT00185393 Goff et al., J Clin Oncol, 2009 (63) R-Zevalin consolidation 186 PFS 38.4 vs. 8.2 months 2.6 months and 2.4 years qPCR III <0.029 NCT0138859 Morschhauser et al., Ann Oncol, 2012 (64) R weekly 4 after ASCT 40 PFS 1.6 years vs. not reached Post induction qPCR II 0.0095 ML17638 trial, NCT01144364 Ladetto et al., Blood, 2013 (11) R-F ND/R maintenance 116 3-year PFS 72% vs. 39% end of therapy End of therapy nPCR, qPCR III <0.007 FOLL05 trial, NCT00774826 Galimberti et al., Clin Cancer Res, 2014 (71) R-CHOP, R-FM or R-CVP 220 3-year PFS 64.3% vs. 53.1%, 66% vs. 41% Post induction, 12 months nPCR, qPCR III <0.08, 3 y <0.015 StiL trial, NCT00991211 Zohren et al., Blood, 2015 (65) R-Benda vs. R-CHOP 114 PFS 8.7 months vs. not reached Post induction qPCR III <0.002 Rambaldi et al., Blood, 2005 (61) 6 CHOP + 4 rituximab 66 FFR 64% vs. 32% Post induction qPCR II 0.006 GALLIUM study, NCT01332968 Pott et al., Blood, 2018 (10) G or R + standard chemotherapy (CHOP, CVP, bendamustin) + 2-year maintenance 634 2.5-year PFS 92.6% vs. 85.2% EOI qPCR confirmed by nPCR III <0.0034 FOLL 12 trial, NCT02063685 Federico et al., Hematol Oncol, 2019 (78) R-CHOP + R maint. vs. R-bendamustin + management according to metabolic and molecular response 790 3-year PFS 84% vs. 68% 3 years qPCR III <0.0001 MRD results from clinical trials at relapse GADOLIN study, NCT01059630 Pott et al., Leukemia, 2020 (8) O-Benda + O maint. vs. Benda alone 228 PFS 35.71 vs. 8.54 months EOI qPCR III <0.0001 MRD, minimal residual disease; ASCT, autologous stem cell transplantation; OS, overall survival; PFS, progression-free survival; FFR, freedom from relapse; EOI, end of induction; qPCR, quantitative real-time PCR; nPCR, nested PCR. - MRD assessment is well suited to precisely dissect the impact of different treatment elements of first-line treatment on the reduction of tumor burden and outcome. This was shown first in randomized trials including ASCT and conventional induction (60) where the improved outcome after high dose chemotherapy including ASCT compared to immunochemotherapy could be lead back to a higher number of clinical and MRD responses (80% of R-HDS patients) compared to only 44% of CHOP-R (P<0.001). MRD response was the strongest independent outcome predictor in this trial. Also after conventional chemo-immunotherapy, the impact of different chemotherapy backbones on the depth of response could be measured by MRD. In the phase 3 Gallium trial, where a conventional chemo-immunotherapy with the glycoengineered antibody Obinutuzumab or the anti-CD20 antibody rituximab was compared in 1,202 FL patients receiving first-line treatment, obinutuzumab based treatment reduced the probability of progression, relapse or death up to 30% (10,62). The prospective MRD analysis revealed the superiority of obinutuzumab combinations already early during the treatment course with higher MRD responses (94.3% vs. 88.9% MRD negativity) at interim staging and at EOT that later translated in an improved outcome. Noteworthy, MRD response translated in a superior PFS and OS independent of treatment arm.
- In the same trial, also the impact of the chemotherapy backbone on MRD response could be determined. MRD analysis revealed a favorable impact of bendamustine and rituximab in terms of MRD clearance with 87% MRD responses in the BM in comparison to only 74% by R-CHOP. Noteworthy, these differences were compensated in the obinutuzumab arm, with a higher overall MRD response rate of 93.2% and 92.7%, respectively (62).
- Immunomodulatory treatment has recently been shown to be comparably efficient as chemoimmunotherapy with respect to response induction, PFS and OS in the phase 3 “RELEVANCE” trial in untreated of FL (Morschhauser NEJM 2018, RELEVANCE trial). MRD studies of a subgroup of 440 patients demonstrated the ability of a chemotherapy-free regimen to induce complete MRD response, MRD negativity at week 24 was reached more frequently in the experimental arm (105/117; 90%) than in the R-Chemo arm (70/90; 77%) (P=0.022). The poor prognostic value in terms of PFS for persistence of molecular disease was observed irrespective of treatment arm (79).
- The role of maintenance therapy with an anti-CD20 antibody after induction regimens is generally accepted for long-term disease control in FL, however MRD reappearance or continuous MRD positivity during maintenance is associated with impending clinical relapse (10,11). This has first been shown by Ladetto and colleagues in 227 elderly FL patients receiving rituximab maintenance after a brief chemo-immunotherapy, MRD negative patients had a 3-year PFS of 72% vs. 39% for those who were still MRD-positive after 8 months (11). Moreover, 3-year PFS was 77% for cases in complete remission (CR)/MRD-negative, 59% for patients in partial remission (PR)/MRD negative, 45% for those in CR but MRD-positive, and only 5% for subjects in PR and MRD-positive, thereby showing that MRD really represents an adjunctive value to the clinical response. In the Gallium trial, where MRD was determined in 4–6 monthly intervals during maintenance, landmark analysis for PFS and MRD status at 4, 8 and 12 months during maintenance showed that independent of the antibody used, the MRD status during maintenance is closely associated with clinical relapse (10). Consequently, the study design of novel treatment strategies should focus on achievement and sustained preservation of MRD response. It is tempting to speculate whether an MRD driven maintenance approach would improve outcome.
- The first trial investigating an MRD driven maintenance approach failed to reach the primary endpoint. The FOLL12 trial is a multicenter, randomized, phase III non inferiority study by the Fondazione Italiana Linfomi, comparing standard vs. response adapted maintenance in patients with previously untreated, intermediate-high risk FL (78). Patients were randomized towards a standard maintenance treatment and an experimental arm, where treatment was conducted with three different treatment schemes according to centrally reviewed metabolic and molecular response. In case of a CMR and MRD response maintenance was skipped, in case of CMR without MRD response patients received 4 weekly rituximab doses, and in case of not achieving a CMR (Deauville score 4–5) a radioimmunotherapy with ibritumomab tiuxetan was given followed by a standard rituximab maintenance was given. The results showed inferior outcome for patients without maintenance with a significant worse 3-year PFS of 68% compared to a PFS of 84% with standard maintenance. These results question not principally the value of an MRD driven treatment strategies, but essentially the concept that MRD response/CMR justifies a treatment reduction. As MRD responders have an excellent outcome when receiving maintenance (78), the issue of MRD driven treatment should in fact not be treatment reduction in responders, but a change of treatment modalities in non-responders, as these patients face clinical relapse. Whether a switch of maintenance treatment improves treatment outcome in MRD positive patients should be a focus of future clinical trials.
- Data for the impact of MRD response at relapse in FL are rare. The only larger prospective trial with systematic MRD assessment is the phase 3 Gadolin trial (8). Patients received obinutuzumab (G) plus bendamustine (Benda) induction followed by G maintenance, or Benda induction alone. Patients achieved MRD response early and obinutuzumab induced a high rate of MRD responses, even in rituximab refractory patients. At midterm staging 41/52 (79%) patients receiving G-Benda were MRD-negative vs. 17/36 (47%) patients receiving Benda alone (P=0.0029). At EOI MRD response increased to 86% in the G-Benda arm, while chemotherapy alone was less effective (30/55, 55%) (P=0.0002). MRD-negative patients at EOI had improved PFS (HR, 0.33; 95% CI: 0.19–0.56; P<0.0001) and OS (HR, 0.39; 95% CI: 0.19–0.78; P=0.008) vs. MRD-positive patients. Obinutuzumab maintenance was effective only in MRD-negative patients and potentially delayed lymphoma regrowth (8).
- 18F-FDG PET-CT and achievement of metabolic response has been described as a reliable predictor (72,73) for prognosis in FL. Additionally, the combination of PET-CT and MRD showed in untreated FL patients from the Gallium trial has a superior prognostic value (12). The CMR or MR at the EOI indicate a prolonged PFS and improved OS. In this subgroup analysis of the Gallium trial CMR was achieved in 89% and MRD negativity in 92%, 94% of the group that achieved CMR were also MRD negative. The group with both criteria being negative the PFS after 2.5 years was 85% in contrast to 69% by the group with both criteria being positive. Patients with only one criteria, CMR or MRD being negative, had a higher risk of progression or death (12).
Expert commentary for application of MRD
The usage of specific techniques for MRD assessment is depending from different aspects, mainly from the context of MRD measurement in a clinical trial, as well as the availability of standardized methods. For using MRD as a surrogate endpoint for prognosis, the MRD technique needs to be sensitive enough to reach reproducibly 10E-4 and it should furthermore be performed by standardized methods in an accredited laboratory. QC rounds assuring reproducibility and reliability of results, as well as standardized data reporting should be a prerequisite for MRD assessment. In the context of the European collaborative network EuroMRD, PCR based MRD assessment has been fully standardized and is broadly available on a high standard in Europe, covering all hematological diseases where clonal IG or TRs serve as MRD targets. QPCR for MRD detection of t(14;18) positive FL is sensitive up to 10E-5, fast, cheap and broadly available and is therefore our method of choice. In our hands, also the more elaborate ASO qPCR targeting IG rearrangements in t(14;18) negative FL turned out to be broadly applicable in a large prospective clinical trial (10,62) with well standardized procedures. Given the reasonable costs, the broad availability and the high importance of information generated by MRD diagnostics, we believe that its use should be strongly encouraged in all clinical trials aiming and obtaining prolonged remissions in FL.
However, considering the time and effort of individual primer design and in particular regulatory aspects for MRD as a surrogate endpoint in clinical trials, IG-NGS gains increasing importance for MRD assessment. In FL, one critical point for a broad application of IG-NGS is the availability of an infiltrated tumor sample at diagnosis required for MRD marker identification. While marker identification by conventional PCR and genescan analysis in random PB and BM is about 85% (10,62), this number drops down to 50% for IG-NGS marker screening. This is mainly due to the polyclonal B-cell background in FL, whereby the tumor infiltration should account for at least 1% of input cell copy numbers to allow clonotype identification based on abundancy of clonotypes. In the context of IG-NGS based MRD assessment in clinical trials, the analysis of diagnostic FFPE samples needs to be prospectively considered to increase the number of patients that can be traced by IG-NGS.
The potential of IG-NGS with respect to a higher sensitivity and a more specific readout at low-level MRD needs to be contrasted to rather costly sequencing consumables and a more elaborate and complex data analysis resulting in higher costs per analysis than qPCR. To assign the method of choice for MRD assessment in a clinical trial, parameters like the number of patients and samples and the clinical application of MRD will be crucial.
Summary and perspectives
Multiple clinical trials have proven that MRD diagnostics is an important method to evaluate treatment efficiency by assessing the depth of response. The achievement of MRD response in indolent lymphomas is not equate with cure from the disease like in acute leukemias, but reflects the individual responsiveness to the treatment applied, associated with the expectation for long-term remission. Consequently, MRD response assessment should be integrated in the design of innovative clinical trials to address the individual risk profile and allow a tailored treatment approach.
For its broad application and as a basis for comparing results from different clinical trials, standardized technical procedures must be defined for multi-center operations, including guidelines for a minimum sensitivity, MRD cutoff levels for risk group identification, practical conditions of application, and reporting of results. This applies in particular for NGS-based MRD, where standardization of technical procedures, bioinformatic evaluation and guidelines for data interpretation are currently being developed.
Validation of MRD diagnostics as endpoint in clinical trials is lacking for most mature B-cell malignancies, however progress in standardized technical procedures might change the situation soon. In the landscape of multiple novel drugs and their approval, an earlier read-out of efficiency and prognosis than PFS would be highly desirable especially in FL with a long-lasting remission.
Currently, MRD-guided treatment decision is still not considered suitable for routine clinical practice and therefore MRD monitoring of individual patients is not recommended outside of clinical trials.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Mark Roschewski and Carla Casulo) for the series “Follicular Lymphoma” published in Annals of Lymphoma. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/aol-21-25). The series “Follicular Lymphoma” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tan D, Horning SJ, Hoppe RT, et al. Improvements in observed and relative survival in follicular grade 1-2 lymphoma during 4 decades: the Stanford University experience. Blood 2013;122:981-7. [Crossref] [PubMed]
- Hiddemann W, Buske C, Dreyling M, et al. Treatment strategies in follicular lymphomas: current status and future perspectives. J Clin Oncol 2005;23:6394-9. [Crossref] [PubMed]
- Casulo C, Byrtek M, Dawson KL, et al. Early Relapse of Follicular Lymphoma After Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone Defines Patients at High Risk for Death: An Analysis From the National LymphoCare Study. J Clin Oncol 2015;33:2516-22. [Crossref] [PubMed]
- Salles G, Seymour JF, Offner F, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet 2011;377:42-51. [Crossref] [PubMed]
- Freeman CL, Kridel R, Moccia AA, et al. Early progression after bendamustine-rituximab is associated with high risk of transformation in advanced stage follicular lymphoma. Blood 2019;134:761-4. [Crossref] [PubMed]
- Buske C, Hoster E, Dreyling M, et al. The Follicular Lymphoma International Prognostic Index (FLIPI) separates high-risk from intermediate- or low-risk patients with advanced-stage follicular lymphoma treated front-line with rituximab and the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) with respect to treatment outcome. Blood 2006;108:1504-8. [Crossref] [PubMed]
- Pastore A, Jurinovic V, Kridel R, et al. Integration of gene mutations in risk prognostication for patients receiving first-line immunochemotherapy for follicular lymphoma: a retrospective analysis of a prospective clinical trial and validation in a population-based registry. Lancet Oncol 2015;16:1111-22. [Crossref] [PubMed]
- Pott C, Sehn LH, Belada D, et al. MRD response in relapsed/refractory FL after obinutuzumab plus bendamustine or bendamustine alone in the GADOLIN trial. Leukemia 2020;34:522-32. [Crossref] [PubMed]
- Galimberti S, Genuardi E, Mazziotta F, et al. The Minimal Residual Disease in Non-Hodgkin's Lymphomas: From the Laboratory to the Clinical Practice. Front Oncol 2019;9:528. [Crossref] [PubMed]
- Pott C, Hoster E, Kehden B, et al. Minimal residual disease response at end of induction and during maintenance correlates with updated outcome in the phase III GALLIUM study of obinutuzumab-or rituximab-based immunochemotherapy in previously untreated follicular lymphoma patients. Blood 2018;132:396. [Crossref]
- Ladetto M, Lobetti-Bodoni C, Mantoan B, et al. Persistence of minimal residual disease in bone marrow predicts outcome in follicular lymphomas treated with a rituximab-intensive program. Blood 2013;122:3759-66. [Crossref] [PubMed]
- 23rd Congress of the European Hematology Association Stockholm, Sweden, June 14-17, 2018. HemaSphere 2018;2:1-1113. [Crossref]
- Ladetto M, Brüggemann M, Monitillo L, et al. Next-generation sequencing and real-time quantitative PCR for minimal residual disease detection in B-cell disorders. Leukemia 2014;28:1299-307. [Crossref] [PubMed]
- Drandi D, Kubiczkova-Besse L, Ferrero S, et al. Minimal Residual Disease Detection by Droplet Digital PCR in Multiple Myeloma, Mantle Cell Lymphoma, and Follicular Lymphoma: A Comparison with Real-Time PCR. J Mol Diagn 2015;17:652-60. [Crossref] [PubMed]
- Pott C. Minimal residual disease detection in mantle cell lymphoma: technical aspects and clinical relevance. Semin Hematol 2011;48:172-84. [Crossref] [PubMed]
- Pott C, Brüggemann M, Ritgen M, et al. MRD detection in B-cell non-Hodgkin lymphomas using Ig gene rearrangements and chromosomal translocations as targets for real-time quantitative PCR. In: Küppers P. editor. Lymphoma. 2nd ed. New York: Humana Press, 2019:199-228.
- Kotrova M, Muzikova K, Mejstrikova E, et al. The predictive strength of next-generation sequencing MRD detection for relapse compared with current methods in childhood ALL. Blood 2015;126:1045-7. [Crossref] [PubMed]
- Kotrova M, Darzentas N, Pott C, et al. Next-generation sequencing technology to identify minimal residual disease in lymphoid malignancies. In: Cobaleda C, Sánchez-García I. editors. Leukemia Stem Cells. New York: Humana Press, 2021:95-111.
- Brüggemann M, Kotrová M, Knecht H, et al. Standardized next-generation sequencing of immunoglobulin and T-cell receptor gene recombinations for MRD marker identification in acute lymphoblastic leukaemia; a EuroClonality-NGS validation study. Leukemia 2019;33:2241-53. [Crossref] [PubMed]
- Böttcher S, Ritgen M, Kneba M. Flow cytometric MRD detection in selected mature B-cell malignancies. In: Küppers P. editor. Lymphoma. Totowa: Humana Press, 2013:149-74.
- Böttcher S, Ritgen M, Pott C, et al. Comparative analysis of minimal residual disease detection using four-color flow cytometry, consensus IgH-PCR, and quantitative IgH PCR in CLL after allogeneic and autologous stem cell transplantation. Leukemia 2004;18:1637-45. [Crossref] [PubMed]
- Rawstron AC, Villamor N, Ritgen M, et al. International standardized approach for flow cytometric residual disease monitoring in chronic lymphocytic leukaemia. Leukemia 2007;21:956-64. [Crossref] [PubMed]
- Cheminant M, Derrieux C, Touzart A, et al. Minimal residual disease monitoring by 8-color flow cytometry in mantle cell lymphoma: an EU-MCL and LYSA study. Haematologica 2016;101:336-45. [Crossref] [PubMed]
- van Dongen JJ, Lhermitte L, Böttcher S, et al. EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia 2012;26:1908-75. [Crossref] [PubMed]
- Jacob PM, Nair RA, Jayasudha AV, et al. Downregulation of CD10 in leukaemic phase of follicular lymphoma: a silent deception. J Hematop 2013;6:65-70. [Crossref]
- Cleary ML, Sklar J. Nucleotide sequence of a t(14;18) chromosomal breakpoint in follicular lymphoma and demonstration of a breakpoint-cluster region near a transcriptionally active locus on chromosome 18. Proc Natl Acad Sci U S A 1985;82:7439-43. [Crossref] [PubMed]
- Weinberg OK, Ai WZ, Mariappan MR, et al. ''Minor'' BCL2 breakpoints in follicular lymphoma: frequency and correlation with grade and disease presentation in 236 cases. J Mol Diagn 2007;9:530-7. [Crossref] [PubMed]
- Buchonnet G, Lenain P, Ruminy P, et al. Characterisation of BCL2-JH rearrangements in follicular lymphoma: PCR detection of 3' BCL2 breakpoints and evidence of a new cluster. Leukemia 2000;14:1563-9. [Crossref] [PubMed]
- Lee MS. Molecular aspects of chromosomal translocation t(14;18). Semin Hematol 1993;30:297-305. [PubMed]
- Evans PA, Pott Ch, Groenen PJ, et al. Significantly improved PCR-based clonality testing in B-cell malignancies by use of multiple immunoglobulin gene targets. Report of the BIOMED-2 Concerted Action BHM4-CT98-3936. Leukemia 2007;21:207-14. [Crossref] [PubMed]
- Donovan JW, Ladetto M, Zou G, et al. Immunoglobulin heavy-chain consensus probes for real-time PCR quantification of residual disease in acute lymphoblastic leukemia. Blood 2000;95:2651-8. [Crossref] [PubMed]
- Brüggemann M, Droese J, Bolz I, et al. Improved assessment of minimal residual disease in B cell malignancies using fluorogenic consensus probes for real-time quantitative PCR. Leukemia 2000;14:1419-25. [Crossref] [PubMed]
- Ladetto M, Donovan JW, Harig S, et al. Real-Time polymerase chain reaction of immunoglobulin rearrangements for quantitative evaluation of minimal residual disease in multiple myeloma. Biol Blood Marrow Transplant 2000;6:241-53. [Crossref] [PubMed]
- Callanan MB, Macintyre E, Delfau-Larue MH, et al. Predictive Power of Early, Sequential MRD Monitoring in Peripheral Blood and Bone Marrow in Patients with Mantle Cell Lymphoma Following Autologous Stem Cell Transplantation with or without Rituximab Maintenance; Final Results from the LyMa-MRD Project, Conducted on Behalf of the Lysa Group. Blood 2020;136:12-3. [Crossref]
- van der Velden VH, Cazzaniga G, Schrauder A, et al. Analysis of minimal residual disease by Ig/TCR gene rearrangements: guidelines for interpretation of real-time quantitative PCR data. Leukemia 2007;21:604-11. [Crossref] [PubMed]
- Drandi D, Alcantara M, Benmaad I, et al. Droplet Digital PCR Quantification of Mantle Cell Lymphoma Follow-up Samples From Four Prospective Trials of the European MCL Network. Hemasphere 2020;4:e347. [Crossref] [PubMed]
- Cavalli M, De Novi LA, Della Starza I, et al. Comparative analysis between RQ-PCR and digital droplet PCR of BCL2/IGH gene rearrangement in the peripheral blood and bone marrow of early stage follicular lymphoma. Br J Haematol 2017;177:588-96. [Crossref] [PubMed]
- Della Starza I, Nunes V, Cavalli M, et al. Comparative analysis between RQ-PCR and digital-droplet-PCR of immunoglobulin/T-cell receptor gene rearrangements to monitor minimal residual disease in acute lymphoblastic leukaemia. Br J Haematol 2016;174:541-9. [Crossref] [PubMed]
- Della Starza I, Nunes V, Lovisa F, et al. Droplet Digital PCR Improves IG-/TR-based MRD Risk Definition in Childhood B-cell Precursor Acute Lymphoblastic Leukemia. Hemasphere 2021;5:e543. [Crossref] [PubMed]
- Pott C, Macintyre E, Delfau MH, et al. Prediction of relapse by standardized IG-based allel-specific qPCR, ddPCR and amplicon NGS for MRD monitoring in mantle cell lymphoma: a comparative analysis by the EU-MCL network. Hematol Oncol 2021; [Crossref]
- Langerak AW, Brüggemann M, Davi F, et al. High-Throughput Immunogenetics for Clinical and Research Applications in Immunohematology: Potential and Challenges. J Immunol 2017;198:3765-74. [Crossref] [PubMed]
- Logan AC, Gao H, Wang C, et al. High-throughput VDJ sequencing for quantification of minimal residual disease in chronic lymphocytic leukemia and immune reconstitution assessment. Proc Natl Acad Sci U S A 2011;108:21194-9. [Crossref] [PubMed]
- Faham M, Zheng J, Moorhead M, et al. Deep-sequencing approach for minimal residual disease detection in acute lymphoblastic leukemia. Blood 2012;120:5173-80. [Crossref] [PubMed]
- Pott C, Knecht H, Herzog A, et al. Standardized IGH-based next-generation sequencing for MRD detection in follicular lymphoma. Blood 2017;130:1491.
- Knecht H, Reigl T, Kotrová M, et al. Quality control and quantification in IG/TR next-generation sequencing marker identification: protocols and bioinformatic functionalities by EuroClonality-NGS. Leukemia 2019;33:2254-65. [Crossref] [PubMed]
- Kosmas C, Stamatopoulos K, Papadaki T, et al. Somatic hypermutation of immunoglobulin variable region genes: focus on follicular lymphoma and multiple myeloma. Immunol Rev 1998;162:281-92. [Crossref] [PubMed]
- Lossos IS, Levy R. Mutation analysis of the 5' noncoding regulatory region of the BCL-6 gene in non-Hodgkin lymphoma: evidence for recurrent mutations and intraclonal heterogeneity. Blood 2000;95:1400-5. [Crossref] [PubMed]
- Lossos IS, Levy R. Higher-grade transformation of follicle center lymphoma is associated with somatic mutation of the 5' noncoding regulatory region of the BCL-6 gene. Blood 2000;96:635-9. [Crossref] [PubMed]
- Van Dongen JJM, Langerak AW, Brüggemann M, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia 2003;17:2257-317. [Crossref] [PubMed]
- Langerak AW, Nadel B, De Torbal A, et al. Unraveling the consecutive recombination events in the human IGK locus. J Immunol 2004;173:3878-88. [Crossref] [PubMed]
- Rawstron AC, Fazi C, Agathangelidis A, et al. A complementary role of multiparameter flow cytometry and high-throughput sequencing for minimal residual disease detection in chronic lymphocytic leukemia: an European Research Initiative on CLL study. Leukemia 2016;30:929-36. [Crossref] [PubMed]
- Ching T, Duncan ME, Newman-Eerkes T, et al. Analytical evaluation of the clonoSEQ Assay for establishing measurable (minimal) residual disease in acute lymphoblastic leukemia, chronic lymphocytic leukemia, and multiple myeloma. BMC Cancer 2020;20:612. [Crossref] [PubMed]
- Thompson PA, Srivastava J, Peterson C, et al. Minimal residual disease undetectable by next-generation sequencing predicts improved outcome in CLL after chemoimmunotherapy. Blood 2019;134:1951-9. [Crossref] [PubMed]
- Rodriguez S, Goicoechea I, Gemenetzi K, et al. Discordances between immunofixation (IFx) and minimal residual disease (MRD) assessment with next-generation flow (NGF) and sequencing (NGS) in patients (Pts) with multiple myeloma (MM): clinical and pathogenic significance. Blood 2020;136:5-6. [Crossref]
- Scheijen B, Meijers RWJ, Rijntjes J, et al. Next-generation sequencing of immunoglobulin gene rearrangements for clonality assessment: a technical feasibility study by EuroClonality-NGS. Leukemia 2019;33:2227-40. [Crossref] [PubMed]
- Bystry V, Reigl T, Krejci A, et al. ARResT/Interrogate: an interactive immunoprofiler for IG/TR NGS data. Bioinformatics 2017;33:435-7. [PubMed]
- Rambaldi A, Lazzari M, Manzoni C, et al. Monitoring of minimal residual disease after CHOP and rituximab in previously untreated patients with follicular lymphoma. Blood 2002;99:856-62. [Crossref] [PubMed]
- Gribben JG, Freedman AS, Neuberg D, et al. Immunologic purging of marrow assessed by PCR before autologous bone marrow transplantation for B-cell lymphoma. N Engl J Med 1991;325:1525-33. [Crossref] [PubMed]
- Corradini P, Astolfi M, Cherasco C, et al. Molecular monitoring of minimal residual disease in follicular and mantle cell non-Hodgkin's lymphomas treated with high-dose chemotherapy and peripheral blood progenitor cell autografting. Blood 1997;89:724-31. [Crossref] [PubMed]
- Ladetto M, De Marco F, Benedetti F, et al. Prospective, multicenter randomized GITMO/IIL trial comparing intensive (R-HDS) versus conventional (CHOP-R) chemoimmunotherapy in high-risk follicular lymphoma at diagnosis: the superior disease control of R-HDS does not translate into an overall survival advantage. Blood 2008;111:4004-13. [Crossref] [PubMed]
- Rambaldi A, Carlotti E, Oldani E, et al. Quantitative PCR of bone marrow BCL2/IgH+ cells at diagnosis predicts treatment response and long-term outcome in follicular non-Hodgkin lymphoma. Blood 2005;105:3428-33. [Crossref] [PubMed]
- Pott C, Hoster E, Kehden B, et al. Minimal residual disease in patients with follicular lymphoma treated with obinutuzumab or rituximab as first-line induction immunochemotherapy and maintenance in the phase 3 GALLIUM study. Blood 2016;128:613. [Crossref]
- Goff L, Summers K, Iqbal S, et al. Quantitative PCR analysis for Bcl-2/IgH in a phase III study of Yttrium-90 Ibritumomab Tiuxetan as consolidation of first remission in patients with follicular lymphoma. J Clin Oncol 2009;27:6094-100. [Crossref] [PubMed]
- Morschhauser F, Recher C, Milpied N, et al. A 4-weekly course of rituximab is safe and improves tumor control for patients with minimal residual disease persisting 3 months after autologous hematopoietic stem-cell transplantation: results of a prospective multicenter phase II study in patients with follicular lymphoma. Ann Oncol 2012;23:2687-95. [Crossref] [PubMed]
- Zohren F, Bruns I, Pechtel S, et al. Prognostic value of circulating Bcl-2/IgH levels in patients with follicular lymphoma receiving first-line immunochemotherapy. Blood 2015;126:1407-14. [Crossref] [PubMed]
- Pott C, Schrader C, Gesk S, et al. Quantitative assessment of molecular remission after high-dose therapy with autologous stem cell transplantation predicts long-term remission in mantle cell lymphoma. Blood 2006;107:2271-8. [Crossref] [PubMed]
- Hirt C, Schüler F, Kiefer T, et al. Rapid and sustained clearance of circulating lymphoma cells after chemotherapy plus rituximab: clinical significance of quantitative t(14;18) PCR monitoring in advanced stage follicular lymphoma patients. Br J Haematol 2008;141:631-40. [Crossref] [PubMed]
- Dührsen U, Hüttmann A, Dürig J. Prognostic significance of molecular remission in follicular lymphoma. J Clin Oncol 2010;28:e613. [Crossref] [PubMed]
- López-Guillermo A, Cabanillas F, McLaughlin P, et al. The clinical significance of molecular response in indolent follicular lymphomas. Blood 1998;91:2955-60. [Crossref] [PubMed]
- Schmidt C, Zoellner AK, Jurinovic V, et al. Chemotherapy-free combination of obinutuzumab and ibrutinib in first LINE treatment of follicular lymphoma. The alternative study by the german low grade lymphoma study group (GLSG). Blood 2018;132:448. [Crossref]
- Galimberti S, Luminari S, Ciabatti E, et al. Minimal residual disease after conventional treatment significantly impacts on progression-free survival of patients with follicular lymphoma: the FIL FOLL05 trial. Clin Cancer Res 2014;20:6398-405. [Crossref] [PubMed]
- Trotman J, Barrington SF, Belada D, et al. Prognostic value of end-of-induction PET response after first-line immunochemotherapy for follicular lymphoma (GALLIUM): secondary analysis of a randomised, phase 3 trial. Lancet Oncol 2018;19:1530-42. [Crossref] [PubMed]
- Trotman J, Fournier M, Lamy T, et al. Positron emission tomography-computed tomography (PET-CT) after induction therapy is highly predictive of patient outcome in follicular lymphoma: analysis of PET-CT in a subset of PRIMA trial participants. J Clin Oncol 2011;29:3194-200. [Crossref] [PubMed]
- Cottereau AS, Versari A, Luminari S, et al. Prognostic model for high-tumor-burden follicular lymphoma integrating baseline and end-induction PET: a LYSA/FIL study. Blood 2018;131:2449-53. [Crossref] [PubMed]
- Delfau-Larue MH, van der Gucht A, Dupuis J, et al. Total metabolic tumor volume, circulating tumor cells, cell-free DNA: distinct prognostic value in follicular lymphoma. Blood Adv 2018;2:807-16. [Crossref] [PubMed]
- Luminari S, Galimberti S, Versari A, et al. Positron emission tomography response and minimal residual disease impact on progression-free survival in patients with follicular lymphoma. A subset analysis from the FOLL05 trial of the Fondazione Italiana Linfomi. Haematologica 2016;101:e66-8. [Crossref] [PubMed]
- Pulsoni A, Starza ID, Frattarelli N, et al. Stage I/II follicular lymphoma: spread of bcl-2/IgH+ cells in blood and bone marrow from primary site of disease and possibility of clearance after involved field radiotherapy. Br J Haematol 2007;137:216-20. [Crossref] [PubMed]
- Federico M, Mannina D, Versari A, et al. Response oriented maintenance therapy in advanced follicular lymphoma. Results of the interim analysis of the FOLL12 trial conducted by the Fondazione Italiana Linfomi. Hematol Oncol 2019;37:153-4. [Crossref]
- Delfau-Larue MH, Boulland ML, Beldi-Ferchiou A, et al. Lenalidomide/rituximab induces high molecular response in untreated follicular lymphoma: LYSA ancillary RELEVANCE study. Blood Adv 2020;4:3217-23. [Crossref] [PubMed]
Cite this article as: Pott C, Wellnitz D, Ladetto M. Minimal residual disease in follicular lymphoma. Ann Lymphoma 2021;5:32.


