
Adoptive cellular immunotherapy for Epstein-Barr virus-associated lymphoproliferative disease
Introduction
Epstein-Barr virus (EBV) is a ubiquitous member of the gamma herpes virus family that is associated with a variety of lymphomas and lymphoproliferative disorders (LPD). It infects more than 90% of the adult population worldwide (1). Primary infection occurs in childhood as an asymptomatic or mild infection and/or may result in a more florid infectious mononucleosis syndrome in teenagers and young adults. In healthy seropositive individuals, virus neutralizing antibodies control the spread of infectious virus particles and EBV-specific, human leukocyte antigen (HLA) class I restricted, CD8+ cytotoxic T lymphocytes (CTL) specific to the early lytic cycle proteins kill cells entering the lytic cycle before they are able to release infectious virus particles (2). Regardless of the initial infection, EBV maintains a lifelong latency in B cells and oral epithelial cells. During primary infection, EBV enters the oropharynx replicating within the epithelial cells and infect transiting B-lymphocytes (primarily due to their expression of CD21 which is the major receptor for the virus). EBV can also infect epithelial cells via transfer from infected B cells and other processes (3). EBV-infected naïve B-lymphocytes express proteins comprising the entire EBV genome including the EBV nuclear antigens EBNA1, EBNA2, EBNA3, and EBNALP, membrane proteins LMP1, and LMP2 as well as BARF1 and two small non-translated ribonucleic acids (RNA) (Type III latency) (Figure 1). EBV-infected B-lymphocytes then enter the lymphoid follicles and downregulate the immunogenic proteins to express less immunogenic type II latency proteins (EBNA1, LMP1 and LMP2) and thus rescue them into the memory compartment where the virus persists in latently infected B-lymphocytes by further downregulating expression of viral proteins so as to become invisible to EBV-specific T-lymphocytes (EBVSTs) (4). The frequency of EBV-infected B cells in a healthy person remains stable [approximately 0.1–50 EBV-infected B lymphocytes per 1,000,000 peripheral blood mononuclear cells (PBMCs)] over their lifetime, controlled at these levels by a potent EBVST response (5,6). Periodic expansion of EBV-infected B-cells requires re-expression of viral antigens which restimulates the EBVST response.
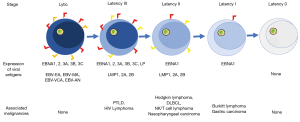
In individuals with weakened immune systems, such as patients with primary immunodeficiency (PID) or infection with human immunodeficiency virus (HIV), recipients of hematopoietic stem cell transplantation (HSCT) or solid organ transplant (SOT), the lack of a robust EBVST response can lead to uncontrolled proliferation of type 3 latency EBV-infected B cells resulting in EBV-associated LPD and malignancies (7). The transformed B-cells in EBV-associated lymphoproliferative disease (EBV-LD) associated with latency type III present several antigenic viral proteins including EBNA 1-3, LMP1 and LMP2 (Figure 1) that induce potent EBVST responses. Such potent T cell immunity maintains the infected B cell pool at <2% of total B cells in immunocompetent individuals but is lacking in immunosuppressed individuals leading to uncontrolled lymphoproliferation of the infected B cells. The scientific rationale for adoptive transfer of EBVSTs is based on harnessing the immunogenicity of type III latency malignancies to control the proliferation of the latently infected B cells. EBV-associated malignancies associated with type II latency [e.g., Hodgkin’s lymphoma (HL), natural killer (NK)/T-cell lymphoma, nasopharyngeal carcinoma (NPC)] or type I latency tumors (e.g., Burkitt lymphoma or gastric carcinoma) can develop in immunocompetent and immune deficient individuals and are much less immunogenic due to downregulation of the immunodominant (e.g., EBNA3 and EBNA2) antigens.
EBV-associated post-transplant lymphoproliferative disease (PTLD) occurs in less than 1% to 25% of HSCT recipients depending on the serostatus of the donor and patient, the degree to which the graft is T-cell depleted, and the post-HSCT immunosuppression (8). In solid organ recipients, incidence of PTLD ranges from 2% to 25% depending on the organ transplanted, passenger lymphocytes in the transplanted organ and type of immunosuppression. However, the biggest risk factor for developing EBV-associated PTLD post-SOT is EBV seronegativity at the time of transplant. Since most children are transplanted at a young age while still being EBV seronegative and convert to EBV seropositivity within 2 years of transplant, EBV driven PTLD is much more common in pediatric SOT recipients (9). More than 90% of EBV-associated PTLD is of mature B-cell origin with cell surface expression of CD20 (10). Therefore, T-cell PTLD is rare and is beyond the scope of this review.
Rituximab, monoclonal antibody targeting CD20 present on the B cells has been an effective monotherapy with response rates of 55% to 100% in HSCT recipients (11-13). Yet, it is limited by increased risk of infection and recurrences. Response rates to rituximab monotherapy in SOT recipients are generally lower and around 50% (9). In pediatric SOT recipients, a combination regimen using low dose cyclophosphamide with prednisone and rituximab has shown event-free survival (EFS) rates of 72% (14). However, these patients with chronic immunosuppression and often impaired organ function have poor chemotherapy tolerance thus there is a need for therapies that address the underlying immune defect, are effective and do not add significant toxicities.
With the expression of multiple immunodominant EBV antigens and the occurrence in the context of immunodeficiency, PTLD is highly immunogenic and amenable to immunotherapy with EBVSTs. Adoptively transferred virus-specific T cells (VSTs) have been evaluated for more than two decades, ranging from the use of donor lymphocyte infusions (DLI) to donor derived multi-antigen specific VSTs in the HSCT setting to autologous as well as allogeneic VSTs in the SOT setting (15-17). However, until relatively recently, this therapy has been available only at specialized centers in the context of single center clinical trials. The objectives of this review are to define current best manufacturing strategies for EBV-specific VSTs, summarize the clinical experience of their use in EBV-related LPD, discuss opportunities to broaden the applicability of this approach and to explore future strategies to enhance their efficacy.
Manufacturing of EBVSTs
EBVSTs can be readily produced from EBV-positive donors using good-manufacturing-grade (GMP) compliant methodologies (Figure 2). Donor types include autologous and allogeneic (including third party) sources which are reviewed in more detail below.
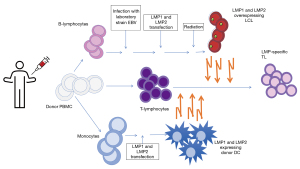
Over the years, several strategies have been developed to manufacture EBVST products with minimal alloreactivity and broad specificity against EBV latency proteins or as a multi-virus specific product with activity against multiple viruses (18-22). The most commonly utilized methods consist of ex vivo expansion of VSTs versus antigen-specific T cell selection [e.g., interferon γ (IFN-γ) capture].
Ex vivo expansion of EBVSTs
Ex vivo expansion of T cells targeting viral antigens via native T cell receptors was established initially by Smith et al. where EBVSTs were selectively expanded utilizing irradiated EBV transformed lymphoblastoid cell lines (LCL) (that express a type III latency pattern of EBV antigen expression) as antigen presenting cells (APCs) to selectively expand EBVSTs (23). LCLs also express high levels of class I and class II HLA and co-stimulatory molecules making them ideal APC for this application (24). LCL-activated EBVSTs consist of a product with activity against early lytic antigens and EBNA 3A, 3B and 3C but unreliable activity towards LMP1 and LMP2 (25,26).
To enhance the specificities to less immunogenic EBV antigens LMP1 and LMP2, several groups have made further modifications to this approach by transducing DC and LCLs with adenovirus vectors to overexpress LMP1 or LMP2 (27). Although this approach helped improve the specificity, the use of LCL and gene engineered APC is complex and time consuming especially when manufacturing from patients (19,28). Subsequently the use of APC such as dendritic cell (DC) pulsed with overlapping peptide libraries spanning whole antigen coupled with the use of artificial APCs [e.g., the novel antigen-presenting complex (KATpx)] can facilitate the rapid (10–21 days as opposed to 2–3 months) expansion of T cells targeting EBNA1, LMP1 and LMP2 from healthy donors and from patients with type 2 latency EBV-associated malignancies (27,29). A further benefit of ex vivo expansion approaches that utilize whole antigen, is that EBVSTs can be manufactured from individuals irrespective of their HLA type. Further, the successful manufacture of EBVSTs derived from EBV seronegative donors has been achieved, including for clinical use, using similar ex vivo expansion methodologies (30,31).
Antigen-specific T cell selection
Other rapid methods include: (I) major histocompatibility complex (MHC) multimer selection where oligomeric forms of MHC molecules are designed and conjugated to magnetic beads to isolate typically CD8+ T cells with high affinity to a specific viral epitope/peptide in an HLA restricted manner (32); (II) IFN-γ capture approaches where mononuclear cells are pulsed with antigen [e.g., single peptides, overlapping peptides (pepmixes), etc.] to isolate CD8+ and CD4+ T cells which secrete IFN-γ in response to the viral antigens (33-35). Although these methodologies can manufacture a product in 48 hours, they require seropositive donors with high frequency of circulating antigen/epitope specific T cells which may be technically challenging in the autologous setting. Moreover, the HLA-restriction requirement coupled with the selection of a purely CD8+ T cell product that lacks CD4+ T cell help (necessary for a sustained immune response) limits the overall applicability of the multimer-selection approach (36). Nevertheless, utilizing both of these strategies, good manufacturing practice (GMP)-grade EBVST products can be rapidly produced from EBV seropositive autologous and allogeneic donors for clinical use.
Post-HSCT donor-derived T cell therapy
Donor lymphocyte infusions
The earliest reported experience of cellular therapy for the treatment of PTLD utilized unmodified DLI derived from the patient’s EBV seropositive HSCT donor which contained effector cells with activity against EBV (37). Although effective in inducing remissions, this therapy carries a significant risk of graft-versus-host disease (GVHD) (19). Strategies such as selective depletion of T-regulatory cells (Tregs) prior to infusion enhances the graft versus lymphoma (GVL) effect while depletion of naïve T cells has been employed to lessen the risk of GVHD (38-40).
Donor-derived EBVSTs
There is extensive reported experience using donor derived EBVSTs for the prevention and treatment of EBV+ PTLD in the post-HSCT setting when the donor is available (17,22,41-47) (Table 1).
Table 1
| Institution | Indication | Number of patients | Serious adverse events | Outcome |
|---|---|---|---|---|
| BCM, Houston, TX, USA (17,42,43,45) | Prophylaxis; treatment | 101; 13 | Recurrence of aGVHD in 8 patients | Prophylaxis: no PTLD; treatment: CR 84.6% |
| MSKCC, New York, NY, USA (22) | Treatment | 19 | None | CR 68%, median follow-up 80 months |
| Karolinska Institute, Stockholm, Sweden (46) | Prophylaxis | 6 | None | Decrease in viral load in 5 patients, 1 death from PTLD |
| Children’s Research Hospital, Kyoto, Japan (47) | Treatment | 1 | None | No response |
aGVHD, acute graft-versus-host disease; BCM, Baylor College of Medicine; CR, complete response; EBV, Epstein-Barr virus; HSCT, hematopoietic stem cell transplantation; MSKCC, Memorial Sloan Kettering Cancer Center; PTLD, post-transplant lymphoproliferative disease; VST, virus-specific T cell.
In the original report using ex vivo expanded EBVSTs from healthy seropositive donors, the team at St. Jude’s Research Hospital treated ten allogeneic HSCT recipients, three with evidence of EBV reactivation and seven at high risk of reactivation (17). This therapy was well tolerated without significant complications with remarkable reduction in EBV viral copy numbers within 4 weeks, including in a patient with immunoblastic lymphoma. None of the 7 patients who received the EBVSTs as prophylaxis had any EBV reactivation or GVHD and there was evidence of persistence of EBVSTs by tracking of genetic markers on the T cells for a median of 10 weeks. This pivotal study established the safety and early evidence of efficacy of donor-derived EBVSTs for the treatment and prophylaxis of PTLD in transplant recipients (17). These clinical outcomes were replicated in a larger study that reported combined data obtained from St. Jude’s (Memphis, TN, USA), Baylor College of Medicine (BCM) (Houston, TX, USA) and Great Ormond Street (London, England). In this report, 114 patients received donor derived EBVSTs after allogeneic HSCT for the treatment and prevention of EBV-related PTLD (43). None of the 101 patients who received this therapy as prophylaxis developed PTLD. Of the 13 patients with active PTLD, 11 patients achieved a complete remission with evidence of VST persistence up to 9 years post-infusion (43). GVHD rates in this study were low with no development of de novo acute GVHD and only 8 of 51 patients developed a recurrence of their acute GVHD all of whom responded to GVHD therapy. Of 108 evaluable patients, there were 13 patients with chronic GVHD but only 2 patients had extensive chronic GVHD. The Memorial Sloan Kettering Cancer Center (MSKCC) group also published their experience using adoptively transferred unselected T cells (i.e., donor lymphocyte infusions) or EBVSTs (22). Overall response rates (ORR) were 72% and 68% with DLI and EBVSTs, respectively. GVHD was occurred in 17% of patients with DLI infusions but was not seen in any of patients who received EBVSTs (22).
Autologous EBVSTs
Administration of autologous EBVSTs has been used for patients with EBV-associated malignancies outside the context of allogeneic HSCT and in SOT recipients with PTLD where donors usually are not available because of the use of cadaveric grafts. In the SOT setting, the production of autologous EBVSTs is technically more challenging because of the ongoing immunosuppression but this can be overcome with modern manufacturing approaches (48). In theory, autologous EBVSTs are preferrable to donor EBVSTs even if available in the post-SOT setting because PTLD is usually of recipient origin and solid organ grafts are not routinely HLA matched to the recipient. However, the challenges in production and the production time leading to delays in initiation of therapy in patients with a rapidly progressive disease impede the routine use of these products in this setting. Comoli et al. from Pavia in Italy reported the treatment of seven SOT recipients treated prophylactically for high EBV viral load with autologous EBVSTs (Table 2) (49). None of the patients developed PTLD. The group at BCM reported 12 SOT recipients treated with autologous EBVSTs (48). While EBVST infusion did not consistently decrease EBV viremia, none of the patients treated prophylactically progressed to PTLD and the two patients with active PTLD achieved a clinical response [one complete response (CR) and one partial response (PR)].
Table 2
| Institution | Indication | Number of patients | Serious adverse events | Outcome |
|---|---|---|---|---|
| BCM, Houston, TX, USA (48) | Prophylaxis; treatment | 10; 2 | None | No PTLD; 1 CR, 1 PR, follow-up 1 year |
| IRCCS Policlinico S. Matteo, Pavia, Italy (49) | Prophylaxis | 7 | None | Reduction in EBV viral load in 5/7 |
BCM, Baylor College of Medicine; CR, complete response; EBV, Epstein-Barr virus; EBVSTs, EBV-specific T-lymphocytes; PR, partial response; PTLD, post-transplant lymphoproliferative disease; SOT, solid organ transplant.
Autologous EBVSTs have also been used to treat type II latency EBV-associated malignancies including HL, T/NK lymphoma and NPC as adjunctive therapy to chemotherapy and/or in relapsed patients. Such tumors are more challenging targets because of the reduced expression of immunogenic viral antigens in these type II latency tumors which express a more restricted array of antigens (e.g., LMP1, LMP2, EBNA1 and BARF1) compared to type III latency tumors.
EBVST products produced by LCL stimulation alone consist of T cells with specificity predominantly towards early lytic antigens and the immunodominant EBNA3 antigens but less activity towards viral antigens expressed in latency type II (25,26). Several groups have however used these products for the treatment of type II latency EBV-associated malignancies (50,51). A pilot study of 14 patients with relapsed EBV+ HL reported 5 patients who maintained a CR for up to 40 months, two of whom had measurable disease at the time of EBVST infusion. One additional patient achieved a PR and five patients had stable disease (SD) (52). For NPC, locoregional disease control was reported in three out of 4 patients with no activity in metastatic disease (51). Other groups reported response rates of 60–70%. Better responses were observed with EBVST products that included T cells with activity against LMP1 and LMP2 (44,53).
As described above, the BCM group subsequently developed a manufacturing process for a LMP2- and LMP1/2-specific T-cell product (18). In two clinical trials using LMP2- and LMP1/2-specific T cells, respectively, production of EBVSTs was successful in 91% of patients with LMP1 and/or LMP2-specificity detected in 66% of products (27,54). A total of 50 patients with EBV-associated HL or Non-Hodgkin lymphoma (NHL) were treated (27). Of 29 patients receiving latent membrane protein (LMP)-specific T cells as adjuvant therapy after HSCT or chemotherapy, 28 patients remained in a complete remission. Thirteen objective responses, notably 11 CRs were observed in 28 patients with active disease at the time of T cell infusion. These are impressive results in a group of patients with mostly type II latency malignancies which are less immunogenic tumors. LMP-specific T-cell infusions were associated with antigen spreading in responders versus non-responders (27). Specifically, there was a significant increase of T cells specific to lymphoma associated (non-viral) antigens melanoma-associated antigen A4 (MAGE-A4), survivin and preferentially expressed antigen of melanoma (PRAME). In one of the larger studies that evaluated LMP-specific T cell therapy for NPC, the group from the Queensland Institute of Medical Research (QIMR) (Brisbane, Australia) reported 16 patients with metastatic NPC receiving adjuvant therapy with LMP1/2-specific T cells and experiencing longer median survivals compared to a control of 8 patients without adoptive cell therapy (523 versus 220 days) (55).
Third-party T cells
Even with “rapid” technologies, patient-specific EBVST product (autologous or allogeneic) manufacture can still be prolonged when procurement times of donor or patient are considered. Further, donor cells may not be available (e.g., recipients of umbilical cord blood transplants or cadaveric organ transplants). Hence, manufacture of patient-specific products may not be possible or may be so delayed that patients with rapidly progressive disease are not able to access these therapies. For these reasons, a readily available “off the shelf” T cell therapy product is desirable.
The first third-party EBVST bank of 60 EBVST products was established by Haque et al. in the United Kingdom (56). Thirty-three transplant recipients (stem cell, 2; heart, 2; kidney, 13; liver, 10; liver and small bowel, 3; lung, 2; heart and lung, 1) with refractory PTLD between the age of 1–76 years received partially HLA matched EBVSTs. HLA matches ranged from 2 to 5/6 HLA alleles and there was a statistically significant association of better outcome with higher HLA matches. Overall, the response rate (CR and PR) was 64% at 5 weeks and 52% at 6 months. Of note, no significant toxicities were observed, alleviating concerns of graft rejection and/or GVHD with this therapy which fueled the broadened applicability of this approach (20,57-62).
The UK group established a cell bank of 25 donors with HLA alleles prevalent at high frequencies in individuals of European descent (58). Of ten patients treated with products from this bank, 8 achieved a CR. There was one report of grade I skin GVHD but otherwise infusions were well tolerated.
Chiou et al. from Birmingham, United Kingdom published their experience in 10 pediatric SOT recipients with PTLD and reported an ORR of 80% (8 out of 10) (61). This favorable response in a pediatric population may indicate differences in the biology of EBV-driven PTLD in this population who is often EBV naïve at the time of transplant and develops PTLD in the earlier post-transplant period compared to the adult population.
The BCM group published extensively on their third-party EBVST products including the use of off-the-shelf multi-VSTs [EBV, cytomegalovirus (CMV), adenovirus, +/− BK virus (BKV) and human herpes virus 6 (HHV6)] products (59,62,63). In a multicenter study led by BCM investigators, utilizing a bank of 33 VST products, 15 products were given to 50 HSCT recipients with severe refractory viral disease after BMT targeting EBV, CMV and/or adenovirus (62). In total, 9 patients receive these third-party multi-VSTs for EBV-associated PTLD. Overall, the response rate to EBV was 66.7% at 6 weeks and only 1 of the responders had a recurrence but achieved a CR with infusion of donor derived EBVSTs. Subsequently, the group established a third-party bank with 59 multi-VST products covering EBV, CMV, adenovirus, BKV and HHV6. In a phase II single center trial, 2 out of 38 patients were treated for EBV reactivation/PTLD and both achieved a CR (59). In both studies, no significant adverse events attributable to the product were observed.
The Memorial Sloan Kettering experience utilizing a third-party cell bank comprising 330 GMP-grade EBVST products was reported by Prockop et al. (20). A total of 46 patients post-HSCT (33) or SOT (13) and PTLD were treated with three weekly infusions of third-party EBVSTs. The ORR was 68% in HSCT recipients and 54% in SOT recipients with a 1-year overall survival of 88.9% in patients with a CR or PR and 81.9% OS in patients who achieved SD. Eleven patients had evidence of central nervous system (CNS) involvement. Of those, 5 achieved a CR and 4 a durable PR suggesting that EBVSTs have activity in the brain. Given the dismal prognosis of CNS PTLD with 3-year progression-free survival rates in the 30% range, EBVSTs represent a promising therapeutic option for this patient population. Based on the HLA typing of 400 patients from the ethnically diverse New York area population, the investigators estimated that an EBVST bank with products restricted by 40 HLA alleles would be sufficient to cover 95% of that population.
Third-party EBVSTs have been mostly used in the post-transplant setting. There has been no published experience in patients with HIV-associated lymphomas because in the modern era of highly active anti-retroviral therapy (HAART) the incidence in the Western word has decreased (64) and the logistical support and specialized experience needed does not make EBVSTs an easily accessible option for the treatment of patients in the developing world.
Surrogate markers of response have been investigated by several groups (52,56,62,65). Prockop et al. reported in their series that a 2 log10 reduction in EBV viral load was indicative of response, however, not all patients treated with third-party EBVST’s had detectable viral loads at start of therapy (20). Leen et al. also showed reduction of EBV viral load correlated with response in HSCT recipients with EBV-associated disease treated with third-party multi virus specific VSTs (62). Moreover, clinical response and reduction of EBV viremia correlated with an increase of EBVSTs (62). However, there is no standardization of EBV viral load measurements by polymerase chain reaction (PCR) making comparisons between different laboratories impossible. EBV viral loads can be measured in plasma or whole blood. Kanakry et al. compared EBV viral load in plasma versus PBMCs and found that cell-free (plasma) EBV copy number quantification was superior to PBMC to predict response in both immunocompetent as well as immunosuppressed individuals with EBV-associated lymphomas or lymphoproliferative disease (65).
Overall, these reports are highly encouraging demonstrating the feasibility of third-party cell banks able to cover a majority of the referred patient population while also achieving impressive response rates (Table 3). These promising results led to the first cell therapy trial run through a cooperative group with the Children’s Oncology Group piloting third-party EBVSTs for the treatment of newly diagnosed PTLD in pediatric SOT recipients (NCT02900976). This study was recently closed and analysis is ongoing. The same group at Children’s National Hospital supplied another multi-center phase I/II consortium study (PBMTC SUP1701, NCT 03475212) with third-party EBVSTs through the Pediatric Bone Marrow Transplant Consortium (66). This trial had two arms, one for pediatric patients with refractory CMV, EBV and or adenoviral infections post-HSCT and another for pediatric patients with PID disorders suffering from refractory viral infections prior to HSCT.
Table 3
| Institution | Specificity | Indication | Number of patients | Serious adverse events | Outcome |
|---|---|---|---|---|---|
| University of Edinburgh, Edinburgh, UK (56) | EBV | Treatment | 2 (HSCT); 31 (SOT) | None | ORR 51.5% (14 CR + 3 PR) at 6 months with 2 subsequent relapses, follow-up 1–7.5 years |
| MSKCC, New York, NY, USA (20) | EBV | Treatment | 33 (HSCT); 13 (SOT) | 1 grade I skin GVHD | ORR 68% (19 CR and 3 PR) in HSCT and 54% (2 CR and 5 PR) in SOT, follow-up 6–115 months |
| Birmingham Woman’s and Children’s Hospital Foundation NHS Trust, Birmingham, UK (58) | EBV | Treatment | 10 (SOT) | None | ORR 80% (7 CR and 1 PR), 5-year OS 85.7% |
| BCM, Houston, TX, USA (62) | Multi-VST | Treatment | 9 (HSCT) | 2 TMA, 1 GI hemorrhage, all deemed unrelated | ORR 66.6% (2 CR and 4 PR) |
CR, complete response; EBV, Epstein-Barr virus; EBVSTs, EBV-specific T-lymphocytes; GI, gastrointestinal; GVHD, graft-versus-host disease; HSCT, hematopoietic stem cell transplantation; MSKCC, Memorial Sloan Kettering Cancer Center; ORR, overall response rate; OS, overall survival; PR, partial response; PTLD, post-transplant lymphoproliferative disease; SOT, solid organ transplant; TMA, thrombotic microangiopathy; VST, virus-specific T cell.
Modification of EBVSTs to enhance activity
Overcoming the immune suppressive effects of TGF-β to enhance EBVST activity in vivo
As previously discussed, the efficacy of EBVSTs is dependent on the expression of viral antigens and limited by the paucity of EBV antigen expression in malignancies of latency type I and II. Furthermore, the immune evasion strategies (e.g., TGF-β secretion) employed by the tumor microenvironment in the immunocompetent host suppresses functional antitumor T cell responses in vivo (67,68). TGF-β can be released into the tumor microenvironment by tumor cells, fibroblasts and immune cells and creates an immunosuppressive environment by impeding T-cell activation, proliferation and migration. In addition, it affects DC and macrophage antigen presentation and chemotaxis. The potential to overcome the immunosuppressive properties of TGF-β has been studied in EBV-positive HL. The Baylor group developed an EBV/LMP-specific T-cell (LST) product expressing a dominant negative TGF-β receptor type II (DNRII) (69). The DNRII-LSTs were resistant to otherwise inhibitory concentrations of TGF-β. In a phase I dose escalating study, 8 patients with relapsed EBV-positive HL were treated with 2 to 12 doses of TGF-β resistant LSTs with 4 of the 7 evaluable patients with active disease achieving clinical responses that were complete and sustained in two patients greater than 4 years post-infusion (70). Moreover, DNRII-LSTs expanded in vivo and could be detected in the peripheral blood from 2 to 51+ months post-infusion.
Calcineurin resistance to enhance EBVSTs
Given the concerns regarding EBVST persistence in patients who require ongoing immune suppression (e.g., patient post-SOT), several groups have explored gene engineering of virus specific T cells to render them resistant to immune suppressive agents including steroids and calcineurin inhibitors (71-74). In one such example of a proof of principle preclinical study, EBVSTs were genetically engineered to express a mutant form of calcineurin thus rendering them calcineurin inhibitor (CNI) resistant (75). In mouse xenograft models bearing human B-cell lymphoma, treatment with CNI-resistant EBVSTs persisted with enhanced activity in the presence of CNI compared to control EBVSTs.
Chimeric antigen receptor T cells
CD19-chimeric antigen T cells (CD19-CART) have shown impressive efficacy in acute B-lymphoblastic leukemia and in B-cell lymphomas (76-80). In addition to viral antigens, EBV-LD expresses a variety of B-cell antigens targetable by CARTs including CD19, CD20 and CD30. However, their use in EBV-lymphoproliferation is limited by the patient’s immunosuppressive state impeding T cell manufacture and the length of production time.
CD19-CART have been administered in three adult SOT recipients with refractory PTLD (81). All patients developed complications to CART therapy including cytokine release syndrome (CRS), neurotoxicity and acute kidney injury. None responded and all ultimately succumbed to their disease. Therefore, while some anecdotal case reports have been published, the wider use of the CART platform for PTLD will likely require an off-the-shelf product (81,82).
Potential for combination strategies administering EBVSTs with other therapeutic modalities to enhance EBVST activity in vivo
Demethylating agents
Newly EBV-infected B-cells express up to 90 viral genes; however rapid CpG-methylation of viral antigens leads to downregulation of viral protein expression and the latency (83-85). Azacytidine and decitabine are potent CpG demethylating agents. In a mouse xenograft of latency type I Burkitt lymphoma, pretreatment with decitabine induced expression of LMP1 and ENBA3 associated with latency type 3 which sensitized tumor cells to subsequent therapy with EBVSTs (86). In contrast, azacytidine did not increase expression of those proteins.
Checkpoint inhibitors
LMP1 has been shown to induce expression of the checkpoint protein PD-L1 in classic HL (CHL) without 9p24.1 alteration (87). When comparing EBV-positive with EBV-negative CHL, EBV-positive CHL had significant higher PD-L1 expression (88). PD-L1 expression scores were inversely correlated with outcome. Similarly, 76–100% of EBV-positive diffuse large B-cell lymphoma (DLBCL) were found to express PD-L1 (89,90). PD-L1 is expressed in 73% of EBV-positive PTLD (87). There have been several trials using checkpoint blockade in DLBCL hinting at single agent activity (91). A phase I study at BCM is exploring combination therapy of checkpoint inhibitors with EBV directed T cells for EBV+ HL and NHL (NCT02973113). Even though there is a rationale for also combining them with EBVSTs for EBV+ PTLD, the risk of graft rejection and autoimmunity limits their use in that setting.
BCL-2 inhibitors
Latently EBV-infected cells inhibit proapoptotic signals thus ensuring immortality (92). LMP1 upregulates the expression of the anti-apoptotic protein BCL-2 (93). The INSERM group showed that the BCL-2 inhibitor ABT-737 induce remission in approximately 70% of mice PTLD xenografts (94). In preclinical, in vitro studies, pretreatment of malignant B-cell lines with the BCL-2 inhibitor venetoclax increased proapoptotic proteins and sensitivity to CD19-CART; however, co-culture and post-treatment adversely affected the number of CART (95). Further studies are needed to elucidate whether there is a role in EBV lymphoproliferation.
Conclusions
The use of adoptive immunotherapy, particularly using third-party “off the shelf” EBVSTs for the treatment of EBV-LD has shown promise in several studies conducted at specialized centers (20,56,62). More recently, EBVSTs have become more widely available including in industry led multi-center studies and consortium and cooperative group studies (20,66). Further work is however needed to create widely available and commercialized third-party cell banks to broaden the applicability of this approach beyond boutique centers. In addition, strategies need to be explored to enhance the anti-tumor activity of EBVST therapies especially for the less immunogenic type I and II latency tumors. Preclinical work and early clinical trials are in process exploring various gene engineering and combination therapy approaches to improve the potency of EBVSTs in vivo.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Christopher P. Fox and Claire Shannon-Lowe) for the series “Lymphoma and Viruses” published in Annals of Lymphoma. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aol.amegroups.com/article/view/10.21037/aol-21-43/coif). The series “Lymphoma and Viruses” was commissioned by the editorial office without any funding or sponsorship. CMB is a co-founder and scientific advisory board member for Catamaran Bio and Mana Therapeutics and is on the Board of Directors of Cabaletta Bio which are companies with no specific interest in EBV specific T cell therapies or EBV+ lymphomas. CMB has intellectual property related to developing virus-specific T therapies. CMB has performed ad hoc consultancy for BMS (BCMA-CAR-T for Myeloma), Roche (B-cell NHL) and Pfizer (ALCL). She served on an ad hoc advisory board for CDR-Life AG on PTLD. She has received royalties from Cellmedica who licensed the LMP-specific T cell technology. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cohen JI. Epstein-Barr virus infection. N Engl J Med 2000;343:481-92. [Crossref] [PubMed]
- Rickinson AB, Moss DJ. Human cytotoxic T lymphocyte responses to Epstein-Barr virus infection. Annu Rev Immunol 1997;15:405-31. [Crossref] [PubMed]
- Shannon-Lowe C, Rowe M. Epstein-Barr virus infection of polarized epithelial cells via the basolateral surface by memory B cell-mediated transfer infection. PLoS Pathog 2011;7:e1001338. [Crossref] [PubMed]
- Lear AL, Rowe M, Kurilla MG, et al. The Epstein-Barr virus (EBV) nuclear antigen 1 BamHI F promoter is activated on entry of EBV-transformed B cells into the lytic cycle. J Virol 1992;66:7461-8. [Crossref] [PubMed]
- Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med 2004;350:1328-37. [Crossref] [PubMed]
- Compagno F, Basso S, Panigari A, et al. Management of PTLD After Hematopoietic Stem Cell Transplantation: Immunological Perspectives. Front Immunol 2020;11:567020. [Crossref] [PubMed]
- Rooney CM, Loftin SK, Holladay MS, et al. Early identification of Epstein-Barr virus-associated post-transplantation lymphoproliferative disease. Br J Haematol 1995;89:98-103. [Crossref] [PubMed]
- Curtis RE, Travis LB, Rowlings PA, et al. Risk of lymphoproliferative disorders after bone marrow transplantation: a multi-institutional study. Blood 1999;94:2208-16. [PubMed]
- Wistinghausen B, Gross TG, Bollard C. Post-transplant lymphoproliferative disease in pediatric solid organ transplant recipients. Pediatr Hematol Oncol 2013;30:520-31. [Crossref] [PubMed]
- Gross TG, Savoldo B, Punnett A. Posttransplant lymphoproliferative diseases. Pediatr Clin North Am 2010;57:481-503. table of contents. [Crossref] [PubMed]
- Kuehnle I, Huls MH, Liu Z, et al. CD20 monoclonal antibody (rituximab) for therapy of Epstein-Barr virus lymphoma after hemopoietic stem-cell transplantation. Blood 2000;95:1502-5. [Crossref] [PubMed]
- Savani BN, Pohlmann PR, Jagasia M, et al. Does peritransplantation use of rituximab reduce the risk of EBV reactivation and PTLPD? Blood 2009;113:6263-4. [Crossref] [PubMed]
- Petropoulou AD, Porcher R, Peffault de Latour R, et al. Increased infection rate after preemptive rituximab treatment for Epstein-Barr virus reactivation after allogeneic hematopoietic stem-cell transplantation. Transplantation 2012;94:879-83. [Crossref] [PubMed]
- Gross TG, Orjuela MA, Perkins SL, et al. Low-dose chemotherapy and rituximab for posttransplant lymphoproliferative disease (PTLD): a Children's Oncology Group Report. Am J Transplant 2012;12:3069-75. [Crossref] [PubMed]
- Walter EA, Greenberg PD, Gilbert MJ, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med 1995;333:1038-44. [Crossref] [PubMed]
- Riddell SR, Watanabe KS, Goodrich JM, et al. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science 1992;257:238-41. [Crossref] [PubMed]
- Rooney CM, Smith CA, Ng CY, et al. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet 1995;345:9-13. [Crossref] [PubMed]
- Bollard CM, Gottschalk S, Helen Huls M, et al. Good manufacturing practice-grade cytotoxic T lymphocytes specific for latent membrane proteins (LMP)-1 and LMP2 for patients with Epstein-Barr virus-associated lymphoma. Cytotherapy 2011;13:518-22. [Crossref] [PubMed]
- Bollard CM, Rooney CM, Heslop HE. T-cell therapy in the treatment of post-transplant lymphoproliferative disease. Nat Rev Clin Oncol 2012;9:510-9. [Crossref] [PubMed]
- Prockop S, Doubrovina E, Suser S, et al. Off-the-shelf EBV-specific T cell immunotherapy for rituximab-refractory EBV-associated lymphoma following transplantation. J Clin Invest 2020;130:733-47. [Crossref] [PubMed]
- O'Reilly RJ, Prockop S, Hasan A, et al. Therapeutic advantages provided by banked virus-specific T-cells of defined HLA-restriction. Bone Marrow Transplant 2019;54:759-64. [Crossref] [PubMed]
- Doubrovina E, Oflaz-Sozmen B, Prockop SE, et al. Adoptive immunotherapy with unselected or EBV-specific T cells for biopsy-proven EBV+ lymphomas after allogeneic hematopoietic cell transplantation. Blood 2012;119:2644-56. [Crossref] [PubMed]
- Smith CA, Ng CY, Heslop HE, et al. Production of genetically modified Epstein-Barr virus-specific cytotoxic T cells for adoptive transfer to patients at high risk of EBV-associated lymphoproliferative disease. J Hematother 1995;4:73-9. [Crossref] [PubMed]
- McLaughlin LP, Gottschalk S, Rooney CM, et al. EBV-Directed T Cell Therapeutics for EBV-Associated Lymphomas. Methods Mol Biol 2017;1532:255-65. [Crossref] [PubMed]
- Steven NM, Annels NE, Kumar A, et al. Immediate early and early lytic cycle proteins are frequent targets of the Epstein-Barr virus-induced cytotoxic T cell response. J Exp Med 1997;185:1605-17. [Crossref] [PubMed]
- Pudney VA, Leese AM, Rickinson AB, et al. CD8+ immunodominance among Epstein-Barr virus lytic cycle antigens directly reflects the efficiency of antigen presentation in lytically infected cells. J Exp Med 2005;201:349-60. [Crossref] [PubMed]
- Bollard CM, Gottschalk S, Torrano V, et al. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. J Clin Oncol 2014;32:798-808. [Crossref] [PubMed]
- Bollard CM, Straathof KC, Huls MH, et al. The generation and characterization of LMP2-specific CTLs for use as adoptive transfer from patients with relapsed EBV-positive Hodgkin disease. J Immunother 2004;27:317-27. [Crossref] [PubMed]
- Ngo MC, Ando J, Leen AM, et al. Complementation of antigen-presenting cells to generate T lymphocytes with broad target specificity. J Immunother 2014;37:193-203. [Crossref] [PubMed]
- Abraham AA, John TD, Keller MD, et al. Safety and feasibility of virus-specific T cells derived from umbilical cord blood in cord blood transplant recipients. Blood Adv 2019;3:2057-68. Erratum in: Blood Adv 2019 Aug 27;3(16):2453. [Crossref] [PubMed]
- Dave H, Luo M, Blaney JW, et al. Toward a Rapid Production of Multivirus-Specific T Cells Targeting BKV, Adenovirus, CMV, and EBV from Umbilical Cord Blood. Mol Ther Methods Clin Dev 2017;5:13-21. [Crossref] [PubMed]
- Neudorfer J, Schmidt B, Huster KM, et al. Reversible HLA multimers (Streptamers) for the isolation of human cytotoxic T lymphocytes functionally active against tumor- and virus-derived antigens. J Immunol Methods 2007;320:119-31. [Crossref] [PubMed]
- Feuchtinger T, Lücke J, Hamprecht K, et al. Detection of adenovirus-specific T cells in children with adenovirus infection after allogeneic stem cell transplantation. Br J Haematol 2005;128:503-9. [Crossref] [PubMed]
- Feucht J, Joachim L, Lang P, et al. Adoptive T-cell transfer for refractory viral infections with cytomegalovirus, Epstein-Barr virus or adenovirus after allogeneic stem cell transplantation. Klin Padiatr 2013;225:164-9. [Crossref] [PubMed]
- Roddie C, Peggs KS. Immunotherapy for transplantation-associated viral infections. J Clin Invest 2017;127:2513-22. [Crossref] [PubMed]
- Bollard CM, Heslop HE. T cells for viral infections after allogeneic hematopoietic stem cell transplant. Blood 2016;127:3331-40. [Crossref] [PubMed]
- Papadopoulos EB, Ladanyi M, Emanuel D, et al. Infusions of donor leukocytes to treat Epstein-Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N Engl J Med 1994;330:1185-91. [Crossref] [PubMed]
- Grant M, Bollard CM. Developing T-cell therapies for lymphoma without receptor engineering. Blood Adv 2017;1:2579-90. [Crossref] [PubMed]
- Nikiforow S, Kim HT, Daley H, et al. A phase I study of CD25/regulatory T-cell-depleted donor lymphocyte infusion for relapse after allogeneic stem cell transplantation. Haematologica 2016;101:1251-9. [Crossref] [PubMed]
- Bleakley M, Heimfeld S, Loeb KR, et al. Outcomes of acute leukemia patients transplanted with naive T cell-depleted stem cell grafts. J Clin Invest 2015;125:2677-89. [Crossref] [PubMed]
- Peggs KS, Verfuerth S, Pizzey A, et al. Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet 2003;362:1375-7. [Crossref] [PubMed]
- Heslop HE, Ng CY, Li C, et al. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat Med 1996;2:551-5. [Crossref] [PubMed]
- Heslop HE, Slobod KS, Pule MA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood 2010;115:925-35. [Crossref] [PubMed]
- Comoli P, Basso S, Zecca M, et al. Preemptive therapy of EBV-related lymphoproliferative disease after pediatric haploidentical stem cell transplantation. Am J Transplant 2007;7:1648-55. [Crossref] [PubMed]
- Rooney CM, Smith CA, Ng CY, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood 1998;92:1549-55. [Crossref] [PubMed]
- Gustafsson A, Levitsky V, Zou JZ, et al. Epstein-Barr virus (EBV) load in bone marrow transplant recipients at risk to develop posttransplant lymphoproliferative disease: prophylactic infusion of EBV-specific cytotoxic T cells. Blood 2000;95:807-14. [Crossref] [PubMed]
- Imashuku S, Goto T, Matsumura T, et al. Unsuccessful CTL transfusion in a case of post-BMT Epstein-Barr virus-associated lymphoproliferative disorder (EBV-LPD). Bone Marrow Transplant 1997;20:337-40. [Crossref] [PubMed]
- Savoldo B, Goss JA, Hammer MM, et al. Treatment of solid organ transplant recipients with autologous Epstein Barr virus-specific cytotoxic T lymphocytes (CTLs). Blood 2006;108:2942-9. [Crossref] [PubMed]
- Comoli P, Labirio M, Basso S, et al. Infusion of autologous Epstein-Barr virus (EBV)-specific cytotoxic T cells for prevention of EBV-related lymphoproliferative disorder in solid organ transplant recipients with evidence of active virus replication. Blood 2002;99:2592-8. [Crossref] [PubMed]
- Bollard CM, Kuehnle I, Leen A, et al. Adoptive immunotherapy for posttransplantation viral infections. Biol Blood Marrow Transplant 2004;10:143-55. [Crossref] [PubMed]
- Louis CU, Straathof K, Bollard CM, et al. Adoptive transfer of EBV-specific T cells results in sustained clinical responses in patients with locoregional nasopharyngeal carcinoma. J Immunother 2010;33:983-90. [Crossref] [PubMed]
- Bollard CM, Aguilar L, Straathof KC, et al. Cytotoxic T lymphocyte therapy for Epstein-Barr virus+ Hodgkin's disease. J Exp Med 2004;200:1623-33. [Crossref] [PubMed]
- Secondino S, Zecca M, Licitra L, et al. T-cell therapy for EBV-associated nasopharyngeal carcinoma: preparative lymphodepleting chemotherapy does not improve clinical results. Ann Oncol 2012;23:435-41. [Crossref] [PubMed]
- Bollard CM, Gottschalk S, Leen AM, et al. Complete responses of relapsed lymphoma following genetic modification of tumor-antigen presenting cells and T-lymphocyte transfer. Blood 2007;110:2838-45. [Crossref] [PubMed]
- Smith C, Tsang J, Beagley L, et al. Effective treatment of metastatic forms of Epstein-Barr virus-associated nasopharyngeal carcinoma with a novel adenovirus-based adoptive immunotherapy. Cancer Res 2012;72:1116-25. [Crossref] [PubMed]
- Haque T, Wilkie GM, Jones MM, et al. Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial. Blood 2007;110:1123-31. [Crossref] [PubMed]
- Gallot G, Vollant S, Saïagh S, et al. T-cell therapy using a bank of EBV-specific cytotoxic T cells: lessons from a phase I/II feasibility and safety study. J Immunother 2014;37:170-9. [Crossref] [PubMed]
- Vickers MA, Wilkie GM, Robinson N, et al. Establishment and operation of a Good Manufacturing Practice-compliant allogeneic Epstein-Barr virus (EBV)-specific cytotoxic cell bank for the treatment of EBV-associated lymphoproliferative disease. Br J Haematol 2014;167:402-10. [Crossref] [PubMed]
- Tzannou I, Papadopoulou A, Naik S, et al. Off-the-Shelf Virus-Specific T Cells to Treat BK Virus, Human Herpesvirus 6, Cytomegalovirus, Epstein-Barr Virus, and Adenovirus Infections After Allogeneic Hematopoietic Stem-Cell Transplantation. J Clin Oncol 2017;35:3547-57. [Crossref] [PubMed]
- Schultze-Florey RE, Tischer S, Kuhlmann L, et al. Dissecting Epstein-Barr Virus-Specific T-Cell Responses After Allogeneic EBV-Specific T-Cell Transfer for Central Nervous System Posttransplant Lymphoproliferative Disease. Front Immunol 2018;9:1475. [Crossref] [PubMed]
- Chiou FK, Beath SV, Wilkie GM, et al. Cytotoxic T-lymphocyte therapy for post-transplant lymphoproliferative disorder after solid organ transplantation in children. Pediatr Transplant 2018; [Crossref] [PubMed]
- Leen AM, Bollard CM, Mendizabal AM, et al. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood 2013;121:5113-23. [Crossref] [PubMed]
- Tzannou I, Watanabe A, Naik S, et al. "Mini" bank of only 8 donors supplies CMV-directed T cells to diverse recipients. Blood Adv 2019;3:2571-80. [Crossref] [PubMed]
- Cobucci RN, Lima PH, de Souza PC, et al. Assessing the impact of HAART on the incidence of defining and non-defining AIDS cancers among patients with HIV/AIDS: a systematic review. J Infect Public Health 2015;8:1-10. [Crossref] [PubMed]
- Kanakry JA, Hegde AM, Durand CM, et al. The clinical significance of EBV DNA in the plasma and peripheral blood mononuclear cells of patients with or without EBV diseases. Blood 2016;127:2007-17. [Crossref] [PubMed]
- Keller MD, Hanley PJ, Zhang N, et al. Third-Party Virus-Specific T-Cell Infusion for Treatment of Refractory Viral Infections: Interim Results from PBTMC SUP1701. Biol Blood Marrow Transplant 2020;26:S89-90. [Crossref]
- Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer 2005;5:263-74. [Crossref] [PubMed]
- Derynck R, Turley SJ, Akhurst RJ. TGFβ biology in cancer progression and immunotherapy. Nat Rev Clin Oncol 2021;18:9-34. [Crossref] [PubMed]
- Foster AE, Dotti G, Lu A, et al. Antitumor activity of EBV-specific T lymphocytes transduced with a dominant negative TGF-beta receptor. J Immunother 2008;31:500-5. [Crossref] [PubMed]
- Bollard CM, Tripic T, Cruz CR, et al. Tumor-Specific T-Cells Engineered to Overcome Tumor Immune Evasion Induce Clinical Responses in Patients With Relapsed Hodgkin Lymphoma. J Clin Oncol 2018;36:1128-39. [Crossref] [PubMed]
- Koukoulias K, Papayanni PG, Georgakopoulou A, et al. "Cerberus" T Cells: A Glucocorticoid-Resistant, Multi-Pathogen Specific T Cell Product to Fight Infections in Severely Immunocompromised Patients. Front Immunol 2021;11:608701. [Crossref] [PubMed]
- Menger L, Gouble A, Marzolini MA, et al. TALEN-mediated genetic inactivation of the glucocorticoid receptor in cytomegalovirus-specific T cells. Blood 2015;126:2781-9. [Crossref] [PubMed]
- Kaeuferle T, Deisenberger L, Jablonowski L, et al. CRISPR-Cas9-Mediated Glucocorticoid Resistance in Virus-Specific T Cells for Adoptive T Cell Therapy Posttransplantation. Mol Ther 2020;28:1965-73. [Crossref] [PubMed]
- Brewin J, Mancao C, Straathof K, et al. Generation of EBV-specific cytotoxic T cells that are resistant to calcineurin inhibitors for the treatment of posttransplantation lymphoproliferative disease. Blood 2009;114:4792-803. [Crossref] [PubMed]
- Ricciardelli I, Blundell MP, Brewin J, et al. Towards gene therapy for EBV-associated posttransplant lymphoma with genetically modified EBV-specific cytotoxic T cells. Blood 2014;124:2514-22. [Crossref] [PubMed]
- Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med 2018;378:439-48. [Crossref] [PubMed]
- Hsieh EM, Rouce RH. Chimeric antigen receptor T cells for mature B-cell lymphoma and Burkitt lymphoma. Hematology Am Soc Hematol Educ Program 2020;2020:487-93. [Crossref] [PubMed]
- Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol 2019;20:31-42. [Crossref] [PubMed]
- Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med 2019;380:45-56. [Crossref] [PubMed]
- Brudno JN, Kochenderfer JN. Chimeric antigen receptor T-cell therapies for lymphoma. Nat Rev Clin Oncol 2018;15:31-46. [Crossref] [PubMed]
- Krishnamoorthy S, Ghobadi A, Santos RD, et al. CAR-T therapy in solid organ transplant recipients with treatment refractory posttransplant lymphoproliferative disorder. Am J Transplant 2021;21:809-14. [Crossref] [PubMed]
- Melilli E, Mussetti A, Linares GS, et al. Acute Kidney Injury Following Chimeric Antigen Receptor T-Cell Therapy for B-Cell Lymphoma in a Kidney Transplant Recipient. Kidney Med 2021;3:665-8. [Crossref] [PubMed]
- Tierney RJ, Kirby HE, Nagra JK, et al. Methylation of transcription factor binding sites in the Epstein-Barr virus latent cycle promoter Wp coincides with promoter down-regulation during virus-induced B-cell transformation. J Virol 2000;74:10468-79. [Crossref] [PubMed]
- Bergbauer M, Kalla M, Schmeinck A, et al. CpG-methylation regulates a class of Epstein-Barr virus promoters. PLoS Pathog 2010;6:e1001114. [Crossref] [PubMed]
- Sinclair AJ. Could Changing the DNA Methylation Landscape Promote the Destruction of Epstein-Barr Virus-Associated Cancers? Front Cell Infect Microbiol 2021;11:695093. [Crossref] [PubMed]
- Dalton T, Doubrovina E, Pankov D, et al. Epigenetic reprogramming sensitizes immunologically silent EBV+ lymphomas to virus-directed immunotherapy. Blood 2020;135:1870-81. [Crossref] [PubMed]
- Green MR, Rodig S, Juszczynski P, et al. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: implications for targeted therapy. Clin Cancer Res 2012;18:1611-8. [Crossref] [PubMed]
- Roemer MG, Advani RH, Ligon AH, et al. PD-L1 and PD-L2 Genetic Alterations Define Classical Hodgkin Lymphoma and Predict Outcome. J Clin Oncol 2016;34:2690-7. [Crossref] [PubMed]
- Chen BJ, Chapuy B, Ouyang J, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res 2013;19:3462-73. [Crossref] [PubMed]
- Menter T, Bodmer-Haecki A, Dirnhofer S, et al. Evaluation of the diagnostic and prognostic value of PDL1 expression in Hodgkin and B-cell lymphomas. Hum Pathol 2016;54:17-24. [Crossref] [PubMed]
- Goodman A, Patel SP, Kurzrock R. PD-1-PD-L1 immune-checkpoint blockade in B-cell lymphomas. Nat Rev Clin Oncol 2017;14:203-20. [Crossref] [PubMed]
- Fitzsimmons L, Kelly GL. EBV and Apoptosis: The Viral Master Regulator of Cell Fate? Viruses 2017;9:339. [Crossref] [PubMed]
- Henderson S, Rowe M, Gregory C, et al. Induction of bcl-2 expression by Epstein-Barr virus latent membrane protein 1 protects infected B cells from programmed cell death. Cell 1991;65:1107-15. [Crossref] [PubMed]
- Robert A, Pujals A, Favre L, et al. The BCL-2 family protein inhibitor ABT-737 as an additional tool for the treatment of EBV-associated post-transplant lymphoproliferative disorders. Mol Oncol 2020;14:2520-32. [Crossref] [PubMed]
- Yang M, Wang L, Ni M, et al. Pre-sensitization of Malignant B Cells Through Venetoclax Significantly Improves the Cytotoxic Efficacy of CD19.CAR-T Cells. Front Immunol 2020;11:608167. [Crossref] [PubMed]
Cite this article as: Wistinghausen B, Dave H, Bollard CM. Adoptive cellular immunotherapy for Epstein-Barr virus-associated lymphoproliferative disease. Ann Lymphoma 2022;6:5.


