
Complete renal and haematological remission in a case of mantle cell lymphoma associated paraneoplastic focal segmental glomerulosclerosis with ibrutinib: a case report and review of literature
Introduction
Hematologic malignancies are associated with a number of paraneoplastic glomerular, tubulointerstitial and renovascular diseases. Paraneoplastic renal manifestations can be the presenting clinical manifestation of an underlying malignancy (1). Minimal change disease (MCD) associated with Hodgkin lymphoma is a classic example of paraneoplastic glomerulonephritis. Membranoproliferative glomerulonephritis and membranous nephropathy associated with chronic lymphocytic leukemia, hairy cell leukemia and non-Hodgkin lymphoma (NHL) constitute other examples of chronic lymphoproliferative disorder (CLPD) associated paraneoplastic glomerulonephritis (2). Paraneoplastic focal segmental glomerulosclerosis (FSGS) has been rarely described in the setting of lymphoreticular malignancies.
Mantle cell lymphoma (MCL) is one of the aggressive varieties of B-cell NHL with high relapse rate and a median survival of 12–15 years (3). There are sporadic reports of FSGS in the background of MCL treated with chemoimmunotherapy with variable outcomes (4,5). Herein, we describe a rare case of MCL associated FSGS who was successfully treated with a chemotherapy free regimen of single agent ibrutinib and continues to be in sustained complete remission with respect to lymphoma and FSGS related proteinuria at a follow-up of 2 years. We present this case in accordance with the CARE reporting checklist (available at https://aol.amegroups.com/article/view/10.21037/aol-22-21/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
A 73-year-old male patient presented to the outpatient department with history of progressive pedal oedema along with frothy urine of 2-month duration. On examination, patient had an ECOG Performance Status 3 with conjunctival pallor and bilateral pitting pedal oedema extending up to the knees. There were palpable bilateral cervical, axillary, and inguinal lymph nodes of size ~3×3 cm. Liver was palpable 2 cm below the right costal margin and spleen was palpable 4 cm below the left costal margin.
Investigations revealed hemoglobin of 9.1 g/dL (normal range, 13–16 g/dL), total leukocyte count of 7,700 cells/µL (normal range, 4,000–11,000/µL), platelet count 334,000/µL. The differential count had 22% polymorphs, 57% lymphocytes, 8% monocytes, 13% eosinophils with 12% atypical lymphocytes (Figure 1A). Serum creatinine was elevated to 1.6 mg/dL (normal range, 0.5 to 1.2 mg/dL). Serum total protein was 4.7 g/dL (normal range, 6.4–8.3 g/dL) with albumin of 1.8 g/dL (normal range, 3.4–4.8 g/dL). Twenty-four-hour urine protein was 11 g with predominant albuminuria. Lactate dehydrogenase was raised to 470 U/L (upper limit of normal 248 U/L). Serology for hepatitis B, hepatitis C, human immunodeficiency virus, anti-nuclear antibody, and anti-phospholipase A2 receptor antibody was negative.
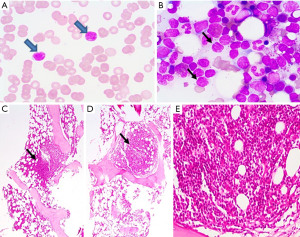
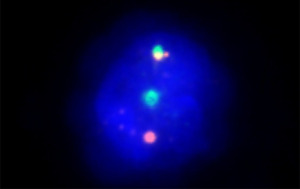
Contrast enhanced computed tomography scan of chest and abdomen showed multiple sub-centimetric lymph nodes in cervical and supraclavicular regions, bilateral para-hilar lymphadenopathy, multiple retroperitoneal, inguinal, and external iliac lymph nodes largest 1.8 cm in size. Bone marrow trephine biopsy imprint smear showed aggregates of atypical lymphocytes (Figure 1B). Bone marrow trephine biopsy showed multiple nodular infiltrates (Figure 1C-1E). On immunohistochemistry the lymphoid nodules were positive for CD 20 and negative for SOX11 with a with a Ki67cell proliferation index ~10%. On multicolor flowcytometry, gated CD19 positive events (11% of viable cells) showed positivity for CD5, CD20, CD79b and surface lambda light chains. They were negative for CD10, CD23, CD43 and surface kappa light chains (Figure 2). Fluorescent in-situ hybridization using dual color break apart probe showed CCND1 rearrangement (Metasystems GmBH; Germany) and was negative for del17p (Figure 3). These findings were consistent with MCL. Lymph node biopsy from right axillary node also showed infiltration by CD20, CD5 and Cyclin D1 positive, intermediate sized atypical lymphoid cells consistent with the diagnosis of MCL.

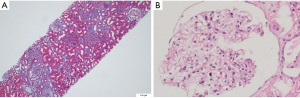
Kidney biopsy showed one of the glomeruli to be globally sclerosed. One other glomerulus showed focal and segmental sclerosis in the para hilar region and synechiae formation. The rest of the glomeruli were unremarkable (Figure 4A,4B). Direct immunofluorescence for immunoglobulin and complement deposits on the kidney tissue was negative. The Congo red stain for amyloid was negative. Based on these findings, he was diagnosed to have MCL Stage IV B with a high-risk Mantle Cell Lymphoma International Prognostic Index (MIPI) score (7.5) and paraneoplastic FSGS.
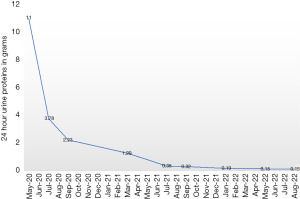
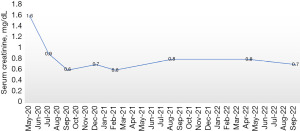
Considering the ongoing COVID-19 pandemic, patient’s age, and his preference for oral therapy he was started on continuous ibrutinib 560 mg per oral daily without any steroids or anti-CD20 directed monoclonal antibodies. An interim F18-fluorodeoxyglucose (FDG) positron emission tomography-computer tomography (PET-CT) scan analysis at 6 months of therapy showed no definite PET evidence of clinically significant abnormal hypermetabolism anywhere in the body. Besides, there was gradual decrease in 24-hour urine protein levels of the patient over a period of 2 years from 11 to 0.15 g (Figure 5). There was also rapid normalization of serum creatinine after the initiation of therapy (Figure 6). At the end of 2 years of follow-up he continues to remain in complete remission for both MCL and nephrotic syndrome due to FSGS.
Discussion
Renal manifestations in MCL are most commonly seen as a result of direct infiltration of the kidney by clonal lymphoid cells, tumor lysis syndrome or nephrotoxic chemotherapeutic drugs. Paraneoplastic membranoproliferative glomerulonephritis and FSGS are rare renal manifestations associated with MCL. Table 1 summarizes the previously published cases of paraneoplastic glomerulonephritides in MCL (4-11). In the two previous cases of FSGS associated with MCL described by Wong et al. and Hindocha et al., FSGS predated the diagnosis of MCL. In both these cases, FSGS directed immunosuppression was not effective, however lymphoma directed chemo-immunotherapy [rituximab, cyclophosphamide, vincristine, prednisolone (R-CVP) and rituximab, cyclophosphamide, hydroxydaunorubicin (doxorubicin), oncovin (vincristine), prednisolone (R-CHOP)] resulted in normalization of renal function and resolution of proteinuria (4,5).
Table 1
| Case | Age (years)/sex | Type of kidney disease | Treatment | Renal outcome | Lymphoma outcome | Reference No. |
|---|---|---|---|---|---|---|
| 1 | 68/M | Focal segmental glomerulonephritis | Short term hemodialysis, steroid, azathioprine, plasma exchange, CVP! | Normalisation of kidney function and proteinuria | Complete remission | (3) |
| 2 | 67/M | Focal segmental glomerulosclerosis | Prednisolone, cyclosporine followed by R-CHOP$ × 6 cycles | Improvement of proteinuria and renal function | Partial remission | (4) |
| 3 | 77/M | Crescentic glomerulonephritis | Short term hemodialysis, CHOP* × 5 cycles, cytarabine × 2 cycles | Partial improvement in renal function and proteinuria | Died while on chemotherapy | (5) |
| 4 | 68/M | Diffuse endocapillary proliferative glomerulonephritis | Hemodialysis, CHP# regimen | Normalisation of kidney function | Complete remission | (6) |
| 5 | 59/– | Membranoproliferative glomerulonephritis, lymphoma infiltration | Short term hemodialysis, cyclophosphamide and steroid | Improvement of kidney function and proteinuria | Clinical response present (further details not available) | (7) |
| 6 | 68/M | Membranoproliferative glomerulonephritis | Short term hemodialysis, rituximab, cyclophosphamide, dexamethasone × 3 cycles followed by H-CVAD¶ | Renal function and proteinuria improved | Not available | (8) |
| 7 | 55/F | Minimal change disease | Steroid × 10 weeks, CHOP* followed by autologous transplant | Normalisation of kidney function and proteinuria | Complete remission | (9) |
| 8 | 77/M | Crescentic glomerulonephritis with lymphoma infiltration | Short-term hemodialysis, CVP! × 6 cycles | Partial improvement in renal function | Died of pulmonary haemorrhage after 3 months, further details not available | (10) |
| 9 | 73/M | Focal segmental glomerulosclerosis | Ibrutinib 560 mg once daily | Normalisation of kidney function and proteinuria | Complete remission | Current case |
!, CVP: cyclophosphamide, vincristine, prednisolone; $, R-CHOP: rituximab, cyclophosphamide, hydroxydaunorubicin (doxorubicin), oncovin (vincristine), prednisolone; *, CHOP: cyclophosphamide, hydroxydaunorubicin (doxorubicin), oncovin (vincristine), prednisolone; #, CHP: cyclophosphamide, hydroxydaunorubicin (doxorubicin), prednisolone; ¶, H-CVAD: hyperfractionated cyclophosphamide, vincristine, adriamycin, dexamethasone. M, male; F, female.
Paraneoplastic glomerulonephritis may be induced by cytokines secreted from tumor cells (12,13). The pathophysiology of paraneoplastic MCD has been studied in detail in Hodgkin lymphoma (14). Increased cytokine levels particularly IL-13, T-helper cell type2 related cytokines are thought to be responsible for the paraneoplastic inflammatory response in Hodgkin lymphoma associated MCD (15). However, primary mechanisms for other B-CLPD associated glomerulonephritis have not been well elucidated. B Cell receptor pathway modulation using targeted BTK inhibitors (BTKi’s) are being increasingly used for managing B-CLPD’s, particularly elderly and frail patients (16,17). In 2013, ibrutinib, a BTKi was first approved by FDA for the treatment of relapsed/refractory MCL (17). Apart from regulating B-cell function, BTK also has role in controlling cytokine production, phagocytosis and formation of inflammatory mediators by other cells of the immune system (18). Ibrutinib irreversibly inhibits BTK-homologous interleukin-2-inducible T-cell kinase, leading to downregulation of Th2 cytokines (19). This particularly explains its role in steroid resistant/refractory chronic graft-versus-host-disease (20).
Recently several studies have suggested the role of Bruton tyrosine kinase pathway upregulation in the pathogenesis of lupus nephritis and IgA nephropathy (21-23). The safety of use of ibrutinib in the setting of lymphoma associated glomerulonephritis needs further studies. Potentially, ibrutinib can not only target the tumor per se, but also accelerate the recovery of glomerulonephritis through its broad spectrum Bruton tyrosine kinase inhibition. The safety of this drug in CLPD associated glomerulonephritis is not well reported, considering the rarity of this diagnosis. While ibrutinib is not excreted by the kidneys, its use has also been associated with worsening of renal function by causing interstitial nephritis, endothelial injury and worsening of hypertension (24,25). However, significant improvement in proteinuria, renal function, and remission of MCL in the current case with the use of ibrutinib suggests its potential safety as well as efficacy in MCL associated paraneoplastic glomerulonephritis (GN).
Conclusions
Our case illustrates an atypical presentation of MCL with nephrotic syndrome due to paraneoplastic FSGS. This case also highlights the potential role of upfront ibrutinib, a Bruton tyrosine kinase inhibitor, in inducing complete remission in MCL and paraneoplastic FSGS, especially in patients unfit for chemotherapy.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://aol.amegroups.com/article/view/10.21037/aol-22-21/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aol.amegroups.com/article/view/10.21037/aol-22-21/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Berns JS, Rosner MH. Onco-nephrology: what the nephrologist needs to know about cancer and the kidney. Clin J Am Soc Nephrol 2012;7:1691. [Crossref] [PubMed]
- Lien YH, Lai LW. Pathogenesis, diagnosis and management of paraneoplastic glomerulonephritis. Nat Rev Nephrol 2011;7:85-95. [Crossref] [PubMed]
- Eskelund CW, Kolstad A, Jerkeman M, et al. 15-year follow-up of the Second Nordic Mantle Cell Lymphoma trial (MCL2): prolonged remissions without survival plateau. Br J Haematol 2016;175:410-8. [Crossref] [PubMed]
- Wong CF, Mohteshamzadeh M, Arsalanizadeh B, et al. Successful treatment of focal segmental glomerulosclerosis in association with mantle cell lymphoma. Ren Fail 2007;29:363-6. [Crossref] [PubMed]
- Hindocha S, Gopaluni S, Collins GP, et al. Focal segmental glomerulosclerosis in a patient with mantle cell lymphoma. BMJ Case Rep 2015;2015:bcr2015211765. [Crossref] [PubMed]
- Rerolle JP, Thervet E, Beaufils H, et al. Crescentic glomerulonephritis and centrocytic lymphoma. Nephrol Dial Transplant 1999;14:1744-5. [Crossref] [PubMed]
- Da'as N, Polliack A, Cohen Y, et al. Kidney involvement and renal manifestations in non-Hodgkin's lymphoma and lymphocytic leukemia: a retrospective study in 700 patients. Eur J Haematol 2001;67:158-64. [Crossref] [PubMed]
- Lubas A, Mróz A, Smoszna J, et al. Membranoproliferative glomerulonephritis, mantle cell lymphoma infiltration, and acute kidney injury. Int Urol Nephrol 2013;45:1489-94. [Crossref] [PubMed]
- Chu JR, Dierksen JE, Glass WF, et al. Association of membranoproliferative glomerulonephritis with mantle cell lymphoma. BMJ Case Rep 2013;2013:bcr2013009730. [Crossref] [PubMed]
- Khow KS, Yong AS, Yong TY, et al. Minimal change disease associated with newly diagnosed mantle cell lymphoma. Ren Fail 2014;36:634-7. [Crossref] [PubMed]
- Miyata KN, Siddiqi NA, Kiss LP, et al. Antineutrophil cytoplasmic antibody-positive pauci-immune glomerulonephritis associated with mantle cell lymphoma. Clin Nephrol Case Stud 2017;5:9-15. [Crossref] [PubMed]
- Ronco PM. Paraneoplastic glomerulopathies: new insights into an old entity. Kidney Int 1999;56:355-77. [Crossref] [PubMed]
- Mallouk A, Pham PT, Pham PC. Concurrent FSGS and Hodgkin's lymphoma: case report and literature review on the link between nephrotic glomerulopathies and hematological malignancies. Clin Exp Nephrol 2006;10:284-9. [Crossref] [PubMed]
- Küppers R, Schwering I, Bräuninger A, et al. Biology of Hodgkin's lymphoma. Ann Oncol 2002;13:11-8. [Crossref] [PubMed]
- Lai KW, Wei CL, Tan LK, et al. Overexpression of interleukin-13 induces minimal-change-like nephropathy in rats. J Am Soc Nephrol 2007;18:1476-85. [Crossref] [PubMed]
- Parikh SA. Chronic lymphocytic leukemia treatment algorithm 2018. Blood Cancer J 2018;8:93. [Crossref] [PubMed]
- Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med 2013;369:507-16. [Crossref] [PubMed]
- Pleyer C, Wiestner A, Sun C. Immunological changes with kinase inhibitor therapy for chronic lymphocytic leukemia. Leuk Lymphoma 2018;59:2792-800. [Crossref] [PubMed]
- Yin Q, Sivina M, Robins H, et al. Ibrutinib Therapy Increases T Cell Repertoire Diversity in Patients with Chronic Lymphocytic Leukemia. J Immunol 2017;198:1740-7. [Crossref] [PubMed]
- Miklos D, Cutler CS, Arora M, et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood 2017;130:2243-50. [Crossref] [PubMed]
- Chalmers SA, Doerner J, Bosanac T, et al. Therapeutic Blockade of Immune Complex-Mediated Glomerulonephritis by Highly Selective Inhibition of Bruton's Tyrosine Kinase. Sci Rep 2016;6:26164. [Crossref] [PubMed]
- Wei J, Wang Y, Qi X, et al. Enhanced Bruton's tyrosine kinase activity in the kidney of patients with IgA nephropathy. Int Urol Nephrol 2021;53:1399-415. [Crossref] [PubMed]
- Ma TK, McAdoo SP, Tam FW. Targeting the tyrosine kinase signalling pathways for treatment of immune-mediated glomerulonephritis: from bench to bedside and beyond. Nephrol Dial Transplant 2017;32:i129-38. [Crossref] [PubMed]
- Markóth C, File I, Szász R, et al. Ibrutinib-induced acute kidney injury via interstitial nephritis. Ren Fail 2021;43:335-9. [Crossref] [PubMed]
- Manohar S, Bansal A, Wanchoo R, et al. Ibrutinib induced acute tubular injury: A case series and review of the literature. Am J Hematol 2019;94:E223-5. [Crossref] [PubMed]
Cite this article as: Bhattacharjee U, Wadhera S, Jain A, Nada R, Sreedharanunni S, Sachdeva MUS, Malhotra P. Complete renal and haematological remission in a case of mantle cell lymphoma associated paraneoplastic focal segmental glomerulosclerosis with ibrutinib: a case report and review of literature. Ann Lymphoma 2023;7:2.


