
Is more better? Increased doses of high dose methotrexate and addition of rituximab is associated with improved outcomes in a large primary CNS lymphoma cohort
Introduction
Primary central nervous system lymphoma (PCNSL) is a rare and aggressive primary brain tumor that is solely confined to the brain, eyes, and cerebrospinal fluid (CSF) and has no systemic involvement. PCNSL compromises only 2% of all primary central nervous system tumors and has an annual incidence rate of 7 cases per 1,000,000 people in the United States, with a rising incidence particularly in the elderly population (1). Based on SEER population data, 5-year overall survival (OS) of immunocompetent individuals is 30.1% [2004–2006], improved from 19.1% [1992–1994] (2).
Over the last several decades, clinical outcomes have improved due to the standard use of high dose methotrexate (HDMTX) containing treatment regimens. HDMTX is given at sufficiently high doses necessary to penetrate the blood brain barrier (BBB) and is the most active single agent that has been identified for treatment of PCNSL. However, while HDMTX based chemotherapy is the backbone of most PCNSL targeted regimens, it remains unclear if the number of HDMTX doses or the addition of rituximab, a monoclonal chimeric antibody targeting CD20 positive B-lymphocytes, has an impact on clinical outcomes.
While rituximab has been shown to improve survival in patients with systemic non-Hodgkin lymphoma, its role in PCNSL and its ability to cross the BBB is less characterized and widely debated. Single arm prospective studies in the relapsed setting as well as many retrospective studies have suggested that rituximab may have some benefit and activity in PCNSL (3-10). However, the randomized prospective HOVON 105/ALLG NHL 24 trial did not detect a difference in event free survival between patients receiving a HDMTX regimen (HDMTX, carmustine, teniposide, oral prednisone) given with or without rituximab followed by consolidation therapy with high dose cytarabine with or without whole brain radiotherapy (11).
In the last 30 years, the standard treatments for PCNSL at Memorial Sloan Kettering Cancer Center (MKSCC) have evolved. HDMTX-containing chemotherapy regimen (mainly HDMTX dosed at 3.5 g/m2, vincristine, and procarbazine) has been predominantly used as mainstay treatment. Around 2006, rituximab was added to this regimen more routinely. Moreover, the number of HDMTX doses administered has increased in recent years. The purpose of this retrospective analysis of a large newly diagnosed PCNSL patient population was to investigate if shifts in standard treatment practices were associated with improvement in clinical outcomes. We present this article in accordance with the STROBE reporting checklist (available at https://aol.amegroups.com/article/view/10.21037/aol-22-19/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This single-center retrospective IRB approved study at MSKCC (No. 16-1398) included patients with immunocompetent PCNSL, age ≥18 years and diagnosed between January 1, 1983 and December 31, 2017. Patients were identified through a departmental database and electronic medical record. Individual consent for this retrospective analysis was waived.
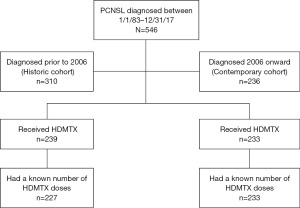
A historic cohort, which was previously reported (12) included patients diagnosed prior to 2006. Patient diagnosed between 2006–2017 served as a contemporary cohort. The contemporary cohort captures a shift in standard treatment for newly diagnosed PCNSL with the addition of rituximab to methotrexate-base chemotherapy and an increase in methotrexate doses from 5 to 8. In both cohorts, standard doses of HDMTX given in our institution remained 3.5 g/m2. Recursive partitioning analysis (RPA) was used to identify previously established and validated prognostic classes (12). The three RPA classes included: class 1 (patients <50 years), class 2 [patients ≥50; Karnofsky performance score (KPS) ≥70] and class 3 (patients ≥50; KPS <70).
The primary objective was to describe OS based on common prognostic markers such as MSKCC prognostic score class (RPA I-III) comprising age and KPS, treatment era, use of rituximab, and number of HDMTX doses.
OS was calculated from the date of diagnosis to death or last follow-up using Kaplan-Meier methodology to visually investigate the effect of treatment era and RPA class on OS. When examining effect of HDMTX on OS in the patients with known HDMTX dosing, OS was calculated from date of last HDMTX dose until death or last follow-up. This was to eliminate the time bias introduced whereby patients who received more doses of HDMTX by definition lived longer to receive the doses. Univariable and multivariable analysis for prognostic factors were analyzed using Cox proportional hazards regression. Multivariable models were adjusted for known or suspected variables associated with OS including treatment, treatment era, sex, and RPA classes. HDMTX and Rituximab were modeled together because there was both a quantitative and qualitative interaction of the two treatments on survival. In a post hoc analysis, multivariable analysis was performed associating prognostic factors with progression-free survival (PFS). PFS was calculated from date of last HDMTX dose until progression, death, or last follow-up, whichever occurred first. Progressions and deaths were considered events and all others were censored. PFS was largely unavailable for the historic cohort. All tests were two-sided with an alpha level of statistical significance set at <0.05.
Statistical analysis
All analyses were performed in R v4.1.3 (The R Foundation for Statistical Computing) and SAS v9.4 (The SAS Institute, Cary, NC, USA). Due to the retrospective nature of this cohort most of the imaging was not available for confirmation of radiographic response and therefore response to first-line therapy was not included in the analysis.
Results
Patient characteristics
Five hundred and forty-six patients with newly diagnosed PCNSL were identified from January 1, 1983 and December 31, 2017. Baseline characteristics are listed in Table 1. Median age at diagnosis was 62 (range, 19 to 90), median KPS at diagnosis was 70 (range, 10 to 100), and 282 (52%) were men. A majority of patients (295, 54%) were classified as RPA class 2.
Table 1
| Characteristics | All (N=546) | Historic (n=310) | Contemporary (n=236) |
|---|---|---|---|
| Age, year: median [range] | 62 [19–90] | 60 [19–89] | 64.5 [21–90] |
| Performance status, KPS: median [range] | 70 [10–100] | 70 [10–100] | 80 [30–100] |
| Gender: male | 282 (51.6) | 160 (51.6) | 122 (51.7) |
| RPA class 1 | 115 (21.1) | 78 (25.2) | 37 (15.7) |
| RPA class 2 | 295 (54.0) | 149 (48.1) | 146 (61.9) |
| RPA class 3 | 136 (24.9) | 83 (26.8) | 53 (22.5) |
| Induction treatment, No. (%) | |||
| HDMTX | 472 (86.5) | 239 (77.1) | 233 (98.7) |
| Unknown % of doses | 12 (2.5) | 12 (5.0) | 0 (0.0) |
| 1–5 HDMTX doses | 284 (60.2) | 195 (81.6) | 89 (38.2) |
| 5 HDMTX doses | 189 (40.0) | 125 (52.3) | 64 (27.5) |
| ≥6 HDMTX doses | 176 (37.3) | 32 (13.4) | 144 (61.8) |
| 6 HDMTX doses | 24 (5.1) | 10 (4.2) | 14 (6.0) |
| 7 HDMTX doses | 74 (15.7) | 20 (8.4) | 54 (23.2) |
| 8+ HDMTX doses | 78 (16.5) | 2 (0.8) | 76 (32.6) |
| Rituximab | 231 (42.3) | 31 (10.0) | 200 (84.7) |
| Consolidation, No. (%) | |||
| WBRT only | 42 (7.7) | 35 (11.3) | 7 (3.0) |
| WBRT + Ara-C | 102 (18.7) | 67 (21.6) | 35 (14.8) |
| Ara-C only | 162 (29.7) | 73 (23.5) | 89 (37.7) |
| ASCT | 55 (10.1) | 7 (2.3) | 48 (20.3) |
| None | 154 (28.2) | 97 (31.3) | 57 (24.2) |
| Unknown | 31 (5.7) | 31 (10.0) | 0 (0) |
| Treated before 2006 (historic cohort) | 310 (56.8) | 310 (100.0) | NA |
| Treated 2006 onward (contemporary cohort) | 236 (43.2) | NA | 236 (100.0) |
KPS, Karnofsky performance score; RPA, recursive partitioning analysis; HDMTX, high dose methotrexate; WBRT, whole brain radiation therapy; Ara-C, cytarabine; ASCT, autologous stem cell transplant; NA, not available.
Patients diagnosed before 2006 (historic control) had a median age of 60 (range, 19–89), median KPS of 70 (range, 10–100) and 52% men compared to patients diagnosed 2006 onward with a median age of 64.5 (range, 21–90), median KPS of 80 (range, 30–100) and 52% men. In both cohorts most patients were classified as RPA class 2.
Initial treatment
In total, 472 patients (86.4%) received HDMTX. The number of HDMTX doses was known in 460: 284 (60.2%) received 1–5 doses (5 doses: 189, 40%) and 176 (37.3%) received 6 or more doses (6 doses: 24, 5.1%, 7 doses: 74, 15.7%, 8+ doses: 78, 16.5%). Based FDA approval and date of diagnosis, 63/546 (11%) patients most likely did not receive granulocyte colony stimulating factor (g-CSF) and 205/546 (38%) did not receive leucovorin. Rituximab was given to 231 (42.3%) patients. Three hundred and ten (56.8%) patients were treated prior to 2006 (historic cohort) and 236 (43.2%) were treated 2006 and afterwards (contemporary cohort). Use of methotrexate chemotherapy was high in both groups (77.1% in the historic and 98.7% in the contemporary cohort) but rituximab use and total methotrexate doses differed. In the historic cohort, only 10% received rituximab and most patients (81.6%) received 1–5 doses of methotrexate. In contrast, 84.8% of patients in the contemporary cohort received rituximab and most patients (61.8%) received ≥6 doses of methotrexate.
Outcomes
Of the 472 patients receiving HDMTX, only those with known number of HDMTX doses (460 patients) were included for outcomes analyses relating to MTX treatment, as seen in Figure 1. The median OS of the entire population was 4.7 years [95% confidence interval (CI): 3.8–5.7 years]; 3.3 years (95% CI: 2.7–3.9 years) in the historic and 8.1 years (95% CI: 6.6–11.1 years) in the contemporary cohort (Figure 2A). The median follow-up for survivors was 2.2 years; 2.5 years for survivors in the historic cohort and 1.8 years for survivors in the contemporary cohort. Five-year OS for the entire study population was 50%; 40.3% (95% CI: 35.1–46.2%) for the historic cohort and 61.8% (95% CI: 55.0–69.5%) for the contemporary cohort (Figure 2A). The median OS in the contemporary cohort was improved in all RPA classes in comparison to patients in the historic cohort. (RPA I: not yet reached vs. 9.2 years; RPA II: 7.6 years vs. 3.5 years; RPA III 2.8 years vs. 1.0 year). For the historic cohort compared to the contemporary cohort, 5-year OS improved from 62.8% to 91.0% in RPA I, 42.0% to 63.0% in RPA II, and 16.3% to 36.9% in RPA III (Figure 2B).
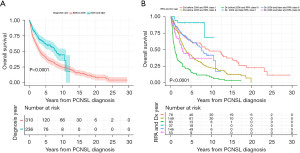
Both the number of HDMTX doses and use of rituximab were associated with improved clinical outcome (Figure 3A-3C). Patients receiving rituximab had improved OS compared with those who did not. Median overall survival (mOS) for patients receiving rituximab was 10.5 years compared to 3.2 years in patients who did not receive rituximab (Figure 3A). The median OS from the last HDMTX dose was 7.8 years in patients receiving 6 or more doses of HDMTX compared to 4.3 years in those receiving less than 6 doses of HDMTX [hazard ratios (HR) =0.63; 95% CI: 0.47–0.83, P=0.001] (Figure 3B). Additionally, there was improved clinical outcome with increased number of HDMTX doses in combination with rituximab use. In patients receiving rituximab, those treated with ≥6 doses of HDMTX had a longer median OS compared to patients receiving <6 doses of HDMTX (13.0 vs. 9.4 years, P=0.001). In contrast, patients not receiving rituximab had a longer median survival of 3.55 years if treated with <6 doses of HDMTX, compared to 1.61 years (P=0.005) in those receiving ≥6 doses (Figure 3C). This was confirmed on multivariable analysis adjusted for treatment era, sex, and RPA classes (Table 2) where ≥6 doses of HDMTX in combination with rituximab was associated with better survival (HR =0.30, 95% CI: 0.19–0.47) in contrast to an increased risk of death (HR =1.56, 95% CI: 1.05–2.33) in those treated with ≥6 doses of HDMTX without rituximab. In a post-hoc analysis associating treatment and PFS in a multivariable model, receiving ≥6 doses of HDMTX and rituximab was also associated with the best PFS (HR =0.24, 95% CI: 0.15–0.38) whereas receiving ≥6 doses of HDMTX without rituximab did not affect PFS (HR =0.94, 95% CI: 0.51–1.74). Multivariable analysis also showed improved survival in patients receiving ≥5 doses of HDMTX and rituximab (HR =0.21, 95% CI: 0.13–0.32, P<0.0001) (Figure S1). Of note, there was no significant difference in clinical outcome in patients receiving <5 HDMTX doses with or without rituximab (Figure S1).

Table 2
| Variable | Level | N (%) | HRa | LCLa | UCLa | Pvala |
|---|---|---|---|---|---|---|
| HDMTX doses and rituximab | 1–5 doses, no rituximab | 196 (42.9) | Ref | – | – | – |
| 1–5 doses, rituximab | 85 (18.6) | 0.53 | 0.36 | 0.80 | 0.002 | |
| 6+ doses, no rituximab | 34 (7.4) | 1.56 | 1.05 | 2.33 | 0.03 | |
| 6+ doses, rituximab | 142 (31.1) | 0.30 | 0.19 | 0.47 | <0.0001 | |
| Treatment era | <2006 | 224 (49.0) | Ref | – | – | – |
| 2006+ | 233 (51.0) | 1.14 | 0.81 | 1.60 | 0.46 | |
| Sex | Male | 236 (51.6) | Ref | – | – | – |
| Female | 221 (48.4) | 0.97 | 0.76 | 1.24 | 0.79 | |
| RPA class | I (<50 years old) | 96 (20.8) | Ref | – | – | – |
| II (50+ years old, 70+ KPS) | 248 (54.3) | 1.99 | 1.39 | 2.86 | 0.0002 | |
| III (50+ years old, <70 KPS) | 114 (25.0) | 4.46 | 3.01 | 6.61 | <0.0001 |
a, adjusted for all variables in the table: treatment, treatment era, sex, and RPA class. N, number; HR, hazard ratio; LCL, lower 95% confidence limit; UCL, upper 95% confidence limit; Pval, P value; HDMTX, high dose methotrexate; Ref, reference; RPA, recursive partitioning analysis; KPS, Karnofsky performance score.
The use of consolidation regimens has changed over time (Table 1). In the historic cohort, whole brain radiation (WBRT) with or without cytarabine was mainly used, whereas in the contemporary group, radiation became obsolete and most patients received cytarabine chemotherapy. High-dose chemotherapy (using thiotepa/busulfan/cyclophosphamide conditioning) with autologous stem cell rescue (ASCT) was nearly ten times more frequently used in the contemporary group (20.3% versus 2.3%). Due to the higher number of patients receiving ASCT in the contemporary cohort and the potential for ASCT associated bias toward better clinical outcomes, we compared the association of MTX doses and use of rituximab in those not receiving ASCT. Multivariable analysis (Table 3) still demonstrated that ≥6 doses of HDMTX in combination with rituximab was associated with better survival (HR =0.28, 95% CI: 0.17–0.45) in contrast to an increased risk of death (HR =1.50, 95% CI: 1.00–2.24) in those treated with ≥6 doses of HDMTX without rituximab.
Table 3
| Variable | Level | N (%) | HRa | LCLa | UCLa | Pvala |
|---|---|---|---|---|---|---|
| HDMTX doses and rituximab | 1–5 doses, no rituximab | 189 (47.0) | Ref | – | – | – |
| 1–5 doses, rituximab | 75 (18.7) | 0.58 | 0.39 | 0.87 | 0.008 | |
| 6+ doses, no rituximab | 34 (8.5) | 1.50 | 1.00 | 2.24 | 0.049 | |
| 6+ doses, rituximab | 104 (25.9) | 0.28 | 0.17 | 0.45 | <0.0001 | |
| Treatment era | <2006 | 217 (54.0) | Ref | – | – | – |
| 2006+ | 185 (46.0) | 1.20 | 0.85 | 1.69 | 0.29 | |
| Sex | Male | 206 (51.2) | Ref | – | – | – |
| Female | 196 (48.8) | 0.97 | 0.75 | 1.24 | 0.79 | |
| RPA class | I (<50 years old) | 75 (18.7) | Ref | – | – | – |
| II (50+ years old, 70+ KPS) | 217 (54.0) | 1.91 | 1.32 | 2.76 | 0.0006 | |
| III (50+ years old, <70 KPS) | 110 (27.4) | 4.03 | 2.70 | 6.03 | <0.0001 |
a, adjusted for all variables in the table: treatment, treatment era, sex, and RPA class. ASCT, autologous stem cell transplant; N, number; HR, hazard ratio; LCL, lower 95% confidence limit; UCL, upper 95% confidence limit; Pval, P value; HDMTX, high dose methotrexate; Ref, reference; RPA, recursive partitioning analysis; KPS, Karnofsky performance score.
Discussion
Our study suggests that OS for newly diagnosed immunocompetent PCNSL has improved over the last few decades. This improvement in OS is consistent with observations in larger population-based studies (1,2). This improvement in OS is largely attributed to the standardization and optimization of HDMTX regimens for treatment of PCNSL.
The RPA classification system, comprising age and KPS cut points, remains a good prognostic tool for newly diagnosed PCNSL. There has been an improvement in OS across all RPA classes in the contemporary cohort. In particular RPA I patients (<50 years of age at diagnosis) had notably improved survival rates, with a median OS not yet reached and 5-year OS of 91%. In addition, RPA III patients (≥50 years and KPS <70) had a more than twice longer survival (2.8 versus 1.0 year) than the historic cohort, further supporting the use of HDMTX-based chemotherapy in frail and elderly patients.
The number of HDMTX doses as well as the use of rituximab seem to have impacted survival rates. HDMTX and Rituximab improved clinical outcomes even after the adjustment for treatment era, suggesting that changes observed are likely due to the modification of HDMTX and Rituximab treatment regimens rather than changes in patient care over time. Additionally, patients who received 6 or more doses of HDMTX had significantly improved survival when also treated with rituximab. This was also true for PFS.
Interestingly, a higher number of HDMTX doses in patients that did not receive rituximab was associated with a poorer clinical outcome. This might be explained by the use of HDMTX in the pre-rituximab era in the historic patient cohort. These patients routinely received 5 doses of HDMTX and for those not achieving a near complete or complete response, additional doses of HDMTX were delivered. Due to this practice pattern, patients receiving >5 HDMTX doses consisted mainly of refractory patients that are at high risk of progression and early death accounting for the poorer outcome in patients receiving a higher number of HDMTX doses but no rituximab. However, it is important to note that this association was accompanied by a wide CI reflecting the small sample size in this subgroup. Additionally, another consideration includes the variability of this population receiving consolidation treatment that may have affected the finding.
In the HOVON 105/ALLG NHL 24 trial (11), the role of rituximab as a component of initial therapy was questioned as there was no significant difference in event free survival when given with the HDMTX based chemotherapy regimen or withheld. Of note, patients in this study received 4 doses of HDMTX. Interestingly, like the HOVON 105/ALG NHL 24 trial, we also did not see a difference in clinical outcome with or without rituximab in those patients receiving <5 doses of HDMTX. In contrast, long-term follow-up of the IELSG32 study (13) showed an improved 7-year OS of 56% in those patients randomized to receive rituximab as part of the MATRix regimen, versus those in non-rituximab containing arms (37% HD-MTX-thiotepa-cytarabine; 21% HD-MTX-cytarabine) supporting a benefit from the addition of rituximab.
There are several limitations to our study that should be taken into consideration. The retrospective nature of this study, limited access to imaging studies to confirm response to first line therapy, the use and efficacy of salvage regimen as well as the single center experience may limit the applicability of these results to a broader population base. On the other hand, HDMTX doses differed minimally. Supportive care was routinely and consistently used since the FDA approval of G-CSF in 1991 and leucovorin in 2002. Sample size and event size in the subgroup receiving ASCT precluded further analyses in this group. Additionally, given the retrospective nature of this study, we cannot exclude inherent patient selection bias. Finally, this study reports the impact of initial HDMTX induction treatment on survival outcomes and did not fully evaluate the impact of consolidation regimens. While a subgroup analysis was performed in patients not receiving ASCT, given considerable present biases, this study cannot assess if differences in consolidation regimens may have affected survival outcomes (14).
Conclusions
In summary, OS for newly diagnosed PCNSL has improved significantly over the last few decades regardless of age, KPS, and RPA class. Patients seem to benefit with the addition of rituximab and when treated with 6 or more doses of HDMTX, an effect independent of treatment era, age, and KPS.
Acknowledgments
Funding: This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748; and also supported by Cycle for Survival Equinox and the Leukemia & Lymphoma Society (to CG).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://aol.amegroups.com/article/view/10.21037/aol-22-19/rc
Data Sharing Statement: Available at https://aol.amegroups.com/article/view/10.21037/aol-22-19/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aol.amegroups.com/article/view/10.21037/aol-22-19/coif). All authors report that this research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748; and also supported by grants from Cycle for Survival Equinox and the Leukemia & Lymphoma Society (to CG). CG reports consultation fees from ONO, BTG, ROCHE and Kite. PY and SNR are employees of Flatiron Health, an independent subsidiary of the Roche Group, and holds stock in Roche. The authors have no other conflicts of interests to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethical board of Memorial Sloan Kettering Cancer Centre (No. 16-1398) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mendez JS, Ostrom QT, Gittleman H, et al. The elderly left behind-changes in survival trends of primary central nervous system lymphoma over the past 4 decades. Neuro Oncol 2018;20:687-94. [Crossref] [PubMed]
- Shiels MS, Pfeiffer RM, Besson C, et al. Trends in primary central nervous system lymphoma incidence and survival in the U.S. Br J Haematol 2016;174:417-24. [Crossref] [PubMed]
- Batchelor TT, Grossman SA, Mikkelsen T, et al. Rituximab monotherapy for patients with recurrent primary CNS lymphoma. Neurology 2011;76:929-30. [Crossref] [PubMed]
- Enting RH, Demopoulos A, DeAngelis LM, et al. Salvage therapy for primary CNS lymphoma with a combination of rituximab and temozolomide. Neurology 2004;63:901-3. [Crossref] [PubMed]
- Gregory G, Arumugaswamy A, Leung T, et al. Rituximab is associated with improved survival for aggressive B cell CNS lymphoma. Neuro Oncol 2013;15:1068-73. [Crossref] [PubMed]
- Holdhoff M, Ambady P, Abdelaziz A, et al. High-dose methotrexate with or without rituximab in newly diagnosed primary CNS lymphoma. Neurology 2014;83:235-9. [Crossref] [PubMed]
- Kansara R, Shenkier TN, Connors JM, et al. Rituximab with high-dose methotrexate in primary central nervous system lymphoma. Am J Hematol 2015;90:1149-54. [Crossref] [PubMed]
- Mocikova H, Pytlik R, Sykorova A, et al. Role of rituximab in treatment of patients with primary central nervous system lymphoma: a retrospective analysis of the Czech lymphoma study group registry. Leuk Lymphoma 2016;57:2777-83. [Crossref] [PubMed]
- Santisteban M, Nieto Y, De la Cruz S, et al. Primary central nervous system lymphoma treated with rituximab plus temozolomide in a second line schedule. Clin Transl Oncol 2007;9:465-7. [Crossref] [PubMed]
- Wong ET, Tishler R, Barron L, et al. Immunochemotherapy with rituximab and temozolomide for central nervous system lymphomas. Cancer 2004;101:139-45. [Crossref] [PubMed]
- Bromberg JEC, Issa S, Bakunina K, et al. Rituximab in patients with primary CNS lymphoma (HOVON 105/ALLG NHL 24): a randomised, open-label, phase 3 intergroup study. Lancet Oncol 2019;20:216-28. [Crossref] [PubMed]
- Abrey LE, Ben-Porat L, Panageas KS, et al. Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol 2006;24:5711-5. [Crossref] [PubMed]
- Ferreri AJM, Cwynarski K, Pulczynski E, et al. Long-term efficacy, safety and neurotolerability of MATRix regimen followed by autologous transplant in primary CNS lymphoma: 7-year results of the IELSG32 randomized trial. Leukemia 2022;36:1870-8. [Crossref] [PubMed]
- Yu J, Du H, Ye X, et al. High-dose methotrexate-based regimens and post-remission consolidation for treatment of newly diagnosed primary CNS lymphoma: meta-analysis of clinical trials. Sci Rep 2021;11:2125. [Crossref] [PubMed]
Cite this article as: Yerram P, Reiss SN, Modelevsky L, Schaff L, Reiner AS, Panageas KS, Grommes C. Is more better? Increased doses of high dose methotrexate and addition of rituximab is associated with improved outcomes in a large primary CNS lymphoma cohort. Ann Lymphoma 2023;7:1.


