
Relapsed mantle cell lymphoma with parenchymal central nervous system involvement successfully treated with chimeric antigen receptor T-cell therapy—a case report
Highlight box
Key findings
• Clonal evolution of indolent mantle cell lymphoma (MCL) with acquisition of blastoid features and TP53 deletion and central nervous system (CNS) relapse.
• Brexucabtagene-autoleucel (Brex-cel, KTE-X19) chimeric antigen receptor T-cell (CAR T) therapy may provide excellent long-lasting remission in relapsed MCL with CNS even in cases with high-risk features with appropriate bridging regimen and post CAR T maintenance such as Bruton tyrosine kinase inhibitor (BTKi).
What is known and what is new?
• MCL with CNS relapse is associated with short survival of about 4 months with the previously existing options such as intrathecal chemotherapy, high dose methotrexate +/− cytarabine, lenalidomide, involved field radiation therapy and ibrutinib or other BTKi.
• Patient in our case report with relapsed MCL patient with CNS involvement remains in remission at 9 months after Brex-cel infusion report with a now on ibrutinib maintenance.
What is the implication, and what should change now?
• We need near-term multicenter “real-world” analyses of outcomes of Brex-cel (KTE-X19) with and without bridging therapy/post CAR T maintenance to validate the outcomes in this case report.
Introduction
Mantle cell lymphoma (MCL) is a distinct subtype of non-Hodgkin lymphoma (NHL) that accounts for approximately 5% of all NHL diagnoses. Outcomes are heterogeneous and are influenced by a number of prognostic factors. Although outcomes generally have improved over the last decade, aggressive variants, complex karyotype, and the presence of p53 mutations or chromosome 17 deletions continue to be associated with poor outcomes.
Central nervous system (CNS) involvement is a rare (0.9–11%) (1-5) complication of MCL, with limited therapeutic options and short survival. CNS MCL with disease relapse is particularly devastating. The median time to CNS relapse in MCL patients after initial therapy is around 15 months, and the median survival after CNS diagnosis is 3.7 months (1,2). There is no standard therapy approach and treatment ranges from intrathecal chemotherapy, high dose methotrexate +/− cytarabine, lenalidomide, involved field radiation therapy and ibrutinib or other Bruton tyrosine kinase inhibitor (BTKi).
Brexucabtagene-autoleucel (Brex-cel, KTE-X19) is a Food and Drug Administration (FDA)-approved chimeric antigen receptor T-cell (CAR T) therapy that specifically targets the CD19 antigen for treating relapsed or refractory (R/R) MCL. Its approval was based on results from the ZUMA-2 trial (6), a phase 2 study involving 74 patients with MCL who had received, on average, three prior treatments including exposure to both chemoimmunotherapy and BTKi. The effectiveness and safety of this CAR T therapy in R/R MCL has been validated by retrospective real-world studies (7-11). In addition to its application in MCL, CAR T therapy has also shown effectiveness and safety in treating patients with diffuse large B cell lymphoma (DLBCL) involving the CNS (12-15). However, in the context of MCL, ZUMA-2 excluded patients with CNS disease (6), and the real-world studies either did not include a specific CNS cohort. Although reports are starting to emerge, the role CAR T in CNS MCL remain limited (11,16).
Here we report a case of a relapsed MCL patient with CNS involvement who remains in remission at 9 months after Brex-cel infusion now on ibrutinib maintenance. We present this case in accordance with the CARE reporting checklist (available at https://aol.amegroups.com/article/view/10.21037/aol-23-22/rc).
Case presentation
A 60-year-old male was first diagnosed with MCL in May 2004. He presented with abdominal pain and was found to have a leukocytosis with a lymphocyte predominance [white blood cell (WBC) 133×109/L] along with massive splenomegaly. Bone marrow examination revealed diffuse infiltration of lymphocytes with co-expression of CD5 and CD19, along with CD23 expression. Fluorescence in situ hybridization (FISH), identified a CCND1/IGH fusion [t(11;14)], indicative of MCL. In addition to splenomegaly, a computed tomography (CT) scan showed enlarged axillary, mesenteric, and retroperitoneal lymph nodes. He was treated with 6 cycles of rituximab, cyclophosphamide, doxorubicin (hydroxydaunorubicin), vincristine (oncovin), prednisone (R-CHOP) and gained complete remission (CR).
In October 2006, right sided cervical adenopathy was identified and an excisional biopsy indicated a recurrence of classical MCL. A bone marrow biopsy showed no lymphoma involvement and a positron emission tomography-CT (PET-CT) scan was negative for other sites of disease.
He began treatment with bortezomib and rituximab, administered weekly for four weeks and two months of weekly bortezomib. By March 2007, he was in CR and initiated maintenance therapy with rituximab and bi-weekly bortezomib which were discontinued in March 2009.
He remained in remission until 2011, when he presented with an isolated right forearm mass and pathology revealed MCL. The mass was resected. There were no significant findings in the marrow and no distant disease and he elected close observation. He remained disease free for 10 years until in December 2022, he presented at an outside hospital with new-onset seizure, word-finding difficulties, and right-sided weakness. Magnetic resonance imaging (MRI) revealed left frontal, right parietal, and right cerebellar masses, a CT showed a left adrenal/periadrenal mass concerning for metastatic disease (Figures 1-3). He underwent a left frontal craniotomy on 12/28/2022 with pathology showing the blastoid variant of MCL. FISH showed CCND1/IGH fusion and deletions of myeloblastosis oncogene (MYB), ataxia telangiectasia mutated (ATM), 13q14.3 and TP53. He was initiated on levetiracetam and dexamethasone with initial improvement of his symptoms. Dexamethasone was then tapered off.
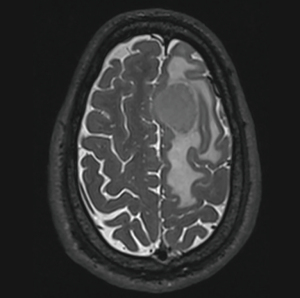


On 11/6/2023, he was started on salvage modified Ferreri protocol chemotherapy (17) (cycle methotrexate =3,500 mg/m2, cytarabine 2,000 mg/m2, and rituximab 375 mg/m2). Brain MRI after 2 cycles revealed responsive disease with persistence of the right cerebellar findings (Figures 4,5). No new disease sites were found. A bone marrow biopsy in early March 2023 was negative for B-cell lymphoma.


After an uneventful T-cell collection and subsequent fludarabine and cyclophosphamide lymphodepleting regimen, he received brexucabtagene (Tecartus) infusion on 3/14/2023 as an inpatient. He did not experience any signs or symptoms of cytokine release syndrome (CRS) or neurotoxicity and was discharged on day 5. He experienced prolonged cytopenia(s). His 30-day post CAR T therapy restaging studies showed CR with no evidence of systemic disease. After adequate platelet (platelet) recovery (platelet >50 k/µL) he initiated ibrutinib 560 mg daily on 5/18/2023. Both days 30 and 90 brain MRIs of the brain demonstrated ongoing response with reduced enhancement at his site of disease. Most recent MRI brain obtained on day 263 revealed ongoing remission. The patient remains in radiological and clinical remission as of day 263 (8 months and 18 days) post CAR T therapy. He continues on ibrutinib with plans for a 1-year adjuvant course.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
We report a unique case of MCL with concomitant CNS and systemic disease, successfully treated with salvage methotrexate and cytarabine chemotherapy followed by CAR T therapy with maintenance ibrutinib. Nine months post-CAR T therapy, the patient remains in CR. Despite experiencing severe cytopenia(s) this patient tolerated CAR T therapy well without CRS or acute neurologic toxicities, possibly mitigated by the salvage therapy associated decreased tumor burden.
MCL with CNS relapse is associated with incredibly poor outcomes, therefore an ongoing CR of 8 months and 18 days is remarkable. Although, the bridging regimen with chemotherapy and post CAR T maintenance with BTKi may be contributing to the remission, neither chemotherapy nor BTKi is expected to induce such a durable remission as seen in this case, suggesting CAR T therapy to be an effective treatment option in MCL with CNS disease. This warrants further analysis with larger multicenter studies.
A recent matched case-control study conducted by McLaughlin et al. revealed that among 36 MCL patients, 11% (4 patients) presented with CNS involvement at the time of diagnosis. These individuals experienced a dismal outcome, with short median overall survival (OS) of 3–4 months compared to those without CNS involvement (2). The median time to CNS involvement typically ranges from 15.2 to 25 months. Remarkably, our case showed a significant delay, with CNS manifestation occurring approximately 20 years after the initial diagnosis.
Advanced stage, blastoid variant, elevated lactate dehydrogenase (LDH), and elevated Ki67 at MCL diagnosis were more common in the CNS MCL cohort (2). Our case also exhibited blastoid features and elevated Ki67, consistent with previous findings on their association with CNS relapse risk (1,2,18). However, a recent study by Conconi et al. only identified blastoid histologic features as a significant predictor (5). B-symptoms, elevated LDH, Eastern Cooperative Oncology Group (ECOG) performance status ≥2, and high Mantle Cell Lymphoma International Prognostic Index (MIPI) scores have been also been suggested as potential CNS involvement risk predictors in MCL, but require further investigation (1). Overall, understanding these predictors may aid in identifying and managing CNS disease in MCL patients. Notably, the patients in the study were treated with non-CAR T-based therapies.
Although the contribution of CNS active agents and ibrutinib maintenance is unclear in this case, data on agents used for management of MCL with CNS involvement is varied. According to Tucker et al. (19), ibrutinib, either as a single agent or in combination with high dose antimetabolites, provided a median duration of response of 4 months. Similarly, Rusconi et al., in a report which included 88 MCL patients with CNS involvement found that ibrutinib was associated with superior OS compared to other chemotherapy agents (20).
Previous studies have shown safety and effectiveness of CAR T therapy in management of DLBCL with CNS involvement (12-15). However, detailed data on safety and effectiveness of CAR T therapy in management of MCL involving CNS is limited to a case report (16). An analysis of 16 patients with relapsed/refractory MCL with CNS involvement from the United States lymphoma CAR T consortium database (4,11) reported an objective response rate (ORR) and CR rate of 81% and 75% respectively. The 12-month progression-free survival (PFS) was 60% and no increased in grade ≥3 neurologic toxicity was seen. Although informative, the details of this cohort were not reported.
Conclusions
In conclusion, our case suggests that indolent MCL may lack high-risk features at diagnosis, but has the potential to clonally evolve by acquiring blastoid features or TP53 deletion leading to development of aggressive MCL with CNS relapse. More importantly, Brex-cel CAR T therapy may provide long lasting remission in relapsed MCL with CNS even in cases with high-risk features when bridged with an appropriate regimen and post CAR T maintenance BTKi. However, further research is required to understand the mechanisms of CAR T effectiveness and to evaluate its success with and without bridging therapy/post CAR T maintenance. In the near-term additional studies, including a detailed, multicenter “real-world” analyses of this population are of particular interest.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://aol.amegroups.com/article/view/10.21037/aol-23-22/rc
Peer Review File: Available at https://aol.amegroups.com/article/view/10.21037/aol-23-22/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aol.amegroups.com/article/view/10.21037/aol-23-22/coif). R.T.M. is an advisor or consultant for Artiva, CRISPR Therapeutics, Incyte, and Novartis; reports honoraria from Bristol Myers Squibb/Celgene, Incyte, and Kite; received research support from Allovir and Novartis; participates in a data and safety monitoring board for Athersys, Century Therapeutics, and VorPharma. S.E.S. is a Distinguished Scholar in Leukemia and Lymphoma Research which provides support for research projects—expert testimony: Abbvie; program research support: Acerta Pharma, Gilead Sciences, Celgene-BMS, Janssen, Profound Bio, Genentech, Beigene, Verastem, Pharmacyclics; paid consultancy: ADCT Therapeutics, Genentec. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cheah CY, George A, Giné E, et al. Central nervous system involvement in mantle cell lymphoma: clinical features, prognostic factors and outcomes from the European Mantle Cell Lymphoma Network. Ann Oncol 2013;24:2119-23. [Crossref] [PubMed]
- McLaughlin N, Wang Y, Witzig T, et al. Central nervous system involvement by mantle cell lymphoma. Leuk Lymphoma 2023;64:371-7. [Crossref] [PubMed]
- Romaguera JE, Medeiros LJ, Hagemeister FB, et al. Frequency of gastrointestinal involvement and its clinical significance in mantle cell lymphoma. Cancer 2003;97:586-91. [Crossref] [PubMed]
- Jain P, Wang Y, Locke FL, et al. Brexucabtagene autoleucel for relapsed/refractory mantle cell lymphoma: Real-world experience from the United States lymphoma CAR T consortium. J Clin Oncol 2022;40:e19583. [Crossref]
- Conconi A, Franceschetti S, Lobetti-Bodoni C, et al. Risk factors of central nervous system relapse in mantle cell lymphoma. Leuk Lymphoma 2013;54:1908-14. [Crossref] [PubMed]
- Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N Engl J Med 2020;382:1331-42. [Crossref] [PubMed]
- Herbaux C, Bret C, Di Blasi R, et al. Kte-X19 in Relapsed or Refractory Mantle-Cell Lymphoma, a "Real-Life" Study from the Descar-T Registry and Lysa Group. Blood 2021;138:743. [Crossref]
- Banerjee T, Newman M, Chen A, et al. A retrospective single-center analysis of CD-19 directed CAR T-cell therapy in relapsed/refractory mantle cell lymphoma. Leuk Lymphoma 2023;64:1472-5. [Crossref] [PubMed]
- Iacoboni G, Rejeski K, Villacampa G, et al. Real-world evidence of brexucabtagene autoleucel for the treatment of relapsed or refractory mantle cell lymphoma. Blood Adv 2022;6:3606-10. [Crossref] [PubMed]
- Heini AD, Bacher U, Kronig MN, et al. Chimeric antigen receptor T-cell therapy for relapsed mantle cell lymphoma: real-world experience from a single tertiary care center. Bone Marrow Transplant 2022;57:1010-2. [Crossref] [PubMed]
- Wang Y, Jain P, Locke FL, et al. Brexucabtagene Autoleucel for Relapsed or Refractory Mantle Cell Lymphoma in Standard-of-Care Practice: Results From the US Lymphoma CAR T Consortium. J Clin Oncol 2023;41:2594-606. [Crossref] [PubMed]
- Alcantara M, Houillier C, Blonski M, et al. CAR T-cell therapy in primary central nervous system lymphoma: the clinical experience of the French LOC network. Blood 2022;139:792-6. [Crossref] [PubMed]
- Ahmed G, Hamadani M, Shah NN. CAR T-cell therapy for secondary CNS DLBCL. Blood Adv 2021;5:5626-30. [Crossref] [PubMed]
- Siddiqi T, Wang X, Blanchard MS, et al. CD19-directed CAR T-cell therapy for treatment of primary CNS lymphoma. Blood Adv 2021;5:4059-63. [Crossref] [PubMed]
- Frigault MJ, Dietrich J, Martinez-Lage M, et al. Tisagenlecleucel CAR T-cell therapy in secondary CNS lymphoma. Blood 2019;134:860-6. [Crossref] [PubMed]
- Vu K, Frank MJ. CAR T-cell therapy for mantle cell lymphoma with central nervous system relapse. Blood Adv 2023;7:375-8. [Crossref] [PubMed]
- Ferreri AJ, Cwynarski K, Pulczynski E, et al. Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol 2016;3:e217-27. [Crossref] [PubMed]
- Chihara D, Asano N, Ohmachi K, et al. Ki-67 is a strong predictor of central nervous system relapse in patients with mantle cell lymphoma (MCL). Ann Oncol 2015;26:966-73. [Crossref] [PubMed]
- Tucker DL, Naylor G, Kruger A, et al. Ibrutinib is a safe and effective therapy for systemic mantle cell lymphoma with central nervous system involvement - a multi-centre case series from the United Kingdom. Br J Haematol 2017;178:327-9. [Crossref] [PubMed]
- Rusconi C, Cheah CY, Eyre TA, et al. Ibrutinib improves survival compared with chemotherapy in mantle cell lymphoma with central nervous system relapse. Blood 2022;140:1907-16. [Crossref] [PubMed]
Cite this article as: Rai M, Maziarz RT, Ratterree B, Bailey ES, Alshalan E, St. Clair PJ, Spurgeon SE. Relapsed mantle cell lymphoma with parenchymal central nervous system involvement successfully treated with chimeric antigen receptor T-cell therapy—a case report. Ann Lymphoma 2024;8:2.


